Early reperfusion for patients with ST-segment elevation myocardial infarction (STEMI) is indicated by the European Society of Cardiology, while a timely invasive strategy is recommended for patients with high-risk and intermediate-risk non-ST-elevation acute coronary syndromes (NSTE-ACS). This study aims to assess patient and system delays according to diagnosis and risk profile, and to identify predictors of prolonged delay.
MethodsWe assembled a cohort of patients (n=939) consecutively admitted to the cardiology department of two hospitals, one in the metropolitan area of Porto and one in the north-east region of Portugal, between August 2013 and December 2014.
ResultsThe proportion of patients with time from symptom onset to first medical contact (FMC) ≥120 min was highest among high-risk NSTE-ACS (57.7%), followed by intermediate-risk NSTE-ACS (52.1%) and STEMI (43.3%). Regardless of diagnosis and risk stratification, use of own transportation and inability to interpret cardiac symptoms correctly were associated with prolonged delays. Regarding system delays, we found that 78.0% of patients with STEMI and 65.8% of patients with high-risk NSTE-ACS were treated in a timeframe exceeding the recommended limits. Admission to a non-percutaneous coronary intervention-capable hospital, admission on weekends and complications at admission were associated with prolonged delays to treatment.
ConclusionsDue to both patient and system delays, a large proportion of STEMI and high-risk NSTE-ACS patients still fail to have access to timely reperfusion.
Atualmente, as normas de orientação clínica da Sociedade Europeia de Cardiologia (SEC) recomendam uma estratégia de reperfusão precoce tanto nos doentes com enfarte agudo do miocárdio com elevação do segmento ST (EAMcST) como nos doentes com síndrome coronária aguda sem elevação do segmento ST (SCAsST) de alto-risco ou risco-intermédio. O objetivo deste estudo foi, considerando o diagnóstico e o perfil de risco dos doentes, avaliar a demora atribuível aos doentes e ao sistema de saúde e identificar preditores de uma demora superior ao recomendado.
MétodosRecrutamos uma coorte de doentes com SCA (n=939) admitidos consecutivamente nos serviços de cardiologia de dois hospitais, um na área metropolitana do Porto e um na região de Trás-os-Montes e Alto Douro, entre agosto de 2013 e dezembro de 2014.
ResultadosA proporção de doentes com tempo ≥120 minutos entre os sintomas e o primeiro contacto com o médico foi de 43,3% nos doentes com EAMcST, 57,7% nos doentes com SCAsST de alto risco e 52,1% nos doentes com SCAsST de risco intermédio. Os doentes que utilizaram transporte próprio e que não reconheceram os sintomas como cardíacos demoraram mais tempo até ao primeiro contacto com o médico. Relativamente aos atrasos atribuíveis ao sistema, 78,0% dos doentes com EAMcST e 65,8% dos doentes com SCAsST de alto-risco demoraram mais tempo do que o recomendado. Ser admitido num hospital sem capacidade de tratamento, ser admitido durante o fim de semana e ter complicações na admissão foram os fatores que contribuíram para os atrasos no tratamento.
ConclusõesOs resultados mostram que uma grande proporção de doentes com SCA é tratada depois do tempo recomendado, limitando o benefício da terapêutica de reperfusão.
Acute coronary syndrome (ACS) remains one of the main causes of death and morbidity in developed countries,1,2 reflecting the limited translation of cardiovascular research achievements into clinical practice. Several factors and processes contribute to this gap between knowledge and practice, some of which is due to treatment delays.
In the management of ACS, the benefits of early reperfusion of patients presenting with ST-segment elevation myocardial infarction (STEMI) are well established.3,4 According to international guidelines, primary percutaneous coronary intervention (PCI) should be performed within 90 min of first medical contact (FMC), and if performed within 120 min of FMC, it is preferable to fibrinolysis.5 However, the optimal reperfusion strategy and timing for patients presenting with non-ST-elevation acute coronary syndromes (NSTE-ACS) are less clear. NSTE-ACS patients are a heterogeneous population, ranging from low-risk patients who may benefit from conservative treatment, to high-risk patients with an increased risk of death and cardiovascular events, who require invasive coronary angiography and revascularization. There is growing evidence supporting the benefits of a routine invasive strategy in patients with a high-risk profile.6,7 However, the optimal timeframe for invasive strategies remains controversial. Despite this, the most recent European Society of Cardiology (ESC) guidelines recommend an immediate invasive strategy (<2 hours) in very high-risk patients, an early invasive strategy (<24 hours) in high-risk patients, and an invasive strategy (<72 hours) in intermediate-risk patients.8
Complying with these guidelines would require the already overburdened healthcare system to provide timely care to more patients, which would then entail reorganization so as to meet these standards and to improve efficiency. Furthermore, since patients are responsible for initiating the cascade of events associated with the treatment pathway, it is also important to analyze patient-related delays. Thus, the purpose of this study is to assess patient and system delays, according to diagnosis and risk profile, and to identify predictors of prolonged treatment delays.
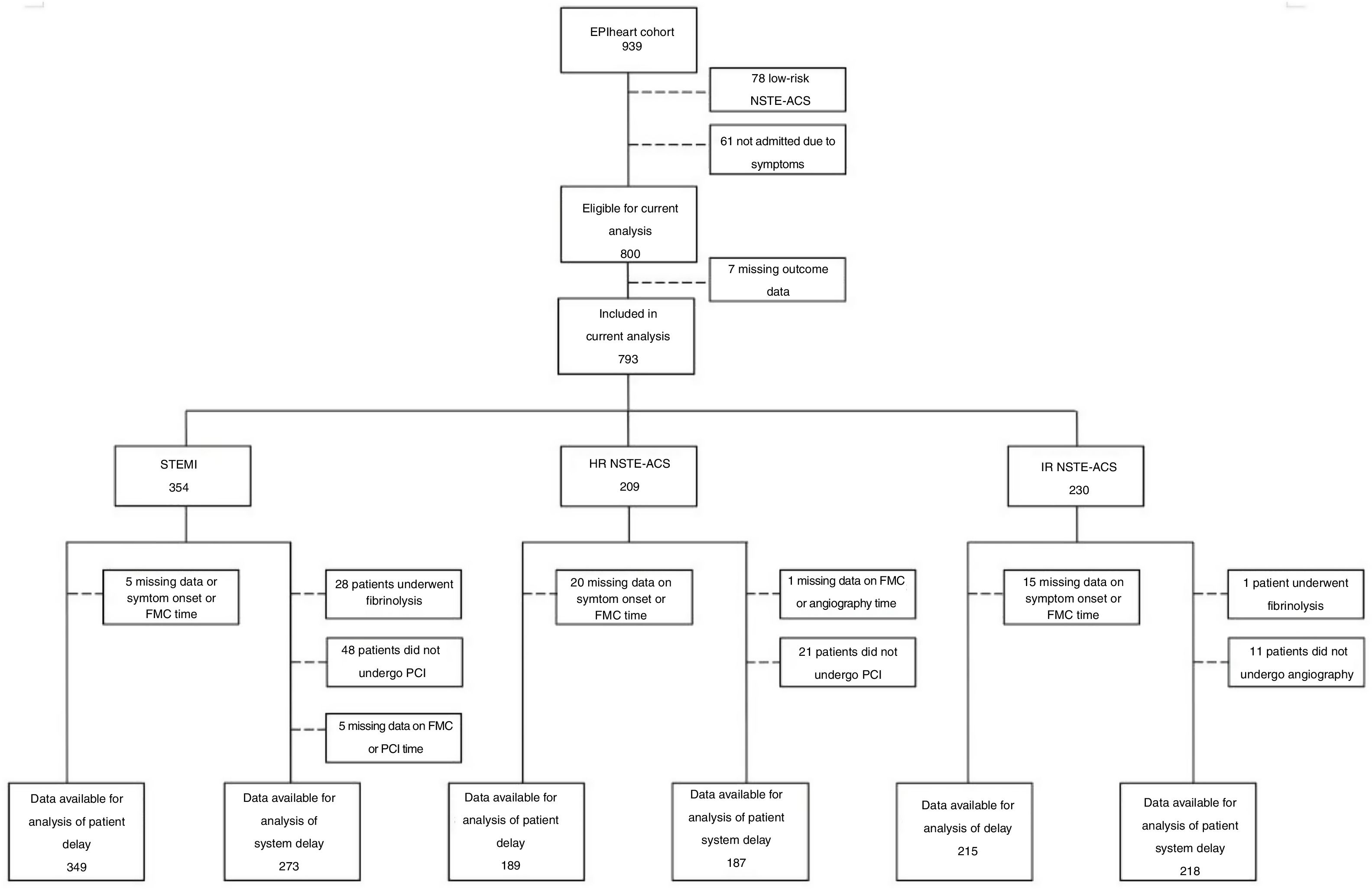
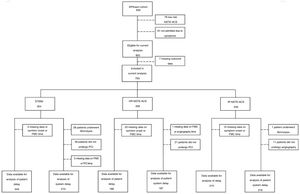
MethodsStudy design and selection of participantsThe EPIHeart project is a prospective cohort assembled in two Portuguese hospitals (Hospital de São João, Porto, and Hospital de São Pedro, north-east region) between August 2013 and December 2014. As described previously,9 the inclusion criteria were admission with a diagnosis of ACS type I, aged ≥18 years, residence in the hospitals’ catchment area, and an expected length of stay longer than 48 hours. Of 1297 patients initially considered, in 164 the diagnosis of ACS was not confirmed and 78 were discharged or transferred or died before study invitation. An additional 44 patients were excluded due to inability to answer the questionnaire (non-Portuguese speaking, hearing problems, or cognitive impairment), as assessed by the interviewer. Seventy-two patients refused to participate (7.1% of those invited), thus, the final study cohort included 939 patients. Non-participants were significantly older (72.7 vs. 64.2 years, p<0.001), more often unpartnered (34.3% vs. 23.1%, p=0.035), and less educated (<4 years of schooling: 43.1% vs. 19.6%, p<0.001), but there was no difference in the proportion of ACS types (STEMI: 37.1% vs. 37.4%, p=0.961). Patients with low-risk NSTE-ACS and those admitted due to physician referral, scheduled medical appointment, or diagnostic exam rather than to symptoms were not included in the analysis. Patients with missing values for time measurements used to determine delays, those who underwent fibrinolysis, and those who did not undergo angiography or PCI were also excluded (Figure 1).
Number of participants for the analyses of patient and system delays, according to diagnosis and risk stratification. FMC: first medical contact; HR: high-risk; IR: intermediate-risk; NSTE-ACS: non-ST-elevation acute coronary syndrome; PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction.
Data were collected through structured interviews and review of medical records. Staff nurses or physicians collected data on clinical presentation and healthcare-seeking behaviors within 48 hours of admission. Chest pain intensity was measured on a visual analog scale (from 0 to 10). Understanding of ACS was assessed by the question: “Did you suspect that your symptoms were related to a cardiac problem?” Symptoms appearing on Saturdays, Sundays, and holidays were defined as symptom onset during weekends. Symptom onset at night was defined as occurring between 9:00 pm and 7:59 am. Next, trained interviewers assessed patients’ sociodemographic characteristics and healthcare use in the previous year. Patients’ place of residence was georeferenced according to their address, using the ArcGIS Online World Geocoding Service and Google Maps. The shortest road distance (in min) from the patient's home to the index hospital was calculated with ArcGIS, version 10.4.1. Patients who were married or in a civil union were considered partnered, while single, separated, divorced, or widowed patients were designated as unpartnered. Health insurance coverage included health subsystems and voluntary private health insurance. Patients were asked about their smoking history and height, and their weight was measured in kg. Other risk factors, medical history, and clinical characteristics were extracted from medical records.
For this analysis, the type of ACS defined in the discharge notes was used and classified as STEMI or NSTE-ACS. Risk stratification defined high-risk patients as those with a Global Registry of Acute Coronary Events (GRACE) score >140; intermediate-risk patients as those with at least one of the following characteristics: diabetes, estimated glomerular filtration rate (eGFR) <60 ml/min, left ventricular ejection fraction (LVEF) <40%, heart failure, prior revascularization, or a GRACE score ≥109 and ≤140; and low-risk patients as those with none of these characteristics.
Time of symptom onset and of FMC were self-reported by patients, while time of hospital admission, coronary angiography, and PCI were gathered from administrative and procedural records. Symptom onset >12 hours was defined according to the time between symptom onset and admission to the index hospital. Patient delay was defined as the time between symptom onset and FMC, whether the latter occurred in a prehospital ambulance, primary healthcare center, hospital, or private clinic. For STEMI patients, system delay was defined as the interval between FMC and PCI, while for NSTE-ACS patients, system delay was defined as the time between hospital admission and coronary angiography.
Statistical analysisData were analyzed considering three groups of patients, defined by their diagnosis and risk stratification: STEMI, high-risk NSTE-ACS, and intermediate-risk NSTE-ACS.
Continuous variables are presented as mean and standard deviation or median and interquartile range (IQR), while categorical variables are reported as frequencies and percentages. Differences between groups were investigated using one-way analysis of variance, the Kruskal-Wallis test, and the chi-square test, as appropriate.
To explore factors associated with prolonged delays, continuous variables capturing time delays were dichotomized. For patient delays a 120-min cut-off was used to define prolonged delays, since there is evidence that reperfusion therapy is of maximum efficacy if given within 120 min of symptom onset.5 For system delays, the ESC recommended cut-off of 90 min was used for STEMI, 24 hours for high-risk NSTE-ACS, and 72 hours for intermediate-risk NSTE-ACS.5,8 Very high-risk NSTE-ACS patients were included in the group of high-risk NSTE-ACS since only one patient was treated within the first two hours. Logistic regression models included all variables that were statistically significant in bivariate analysis (p<0.05). Age, gender, region, and distance were forced into the models.
In order to assess the impact of modifiable variables on delays, population attributable fractions and corresponding 95% confidence interval (CI) were computed. Three alternative scenarios were considered according to the predictors identified in the multivariate models and their potential for change: (1) ambulance transportation of all patients; (2) attribution of chest pain to the heart by all patients; (3) admission of all patients to a PCI-capable hospital.
Statistical analyses were performed using STATA version 11 for Windows (StataCorp LP, College Station, TX).
EthicsThe research protocol was approved by the Ethics Committees at both hospitals. Informed consent was obtained from all patients.
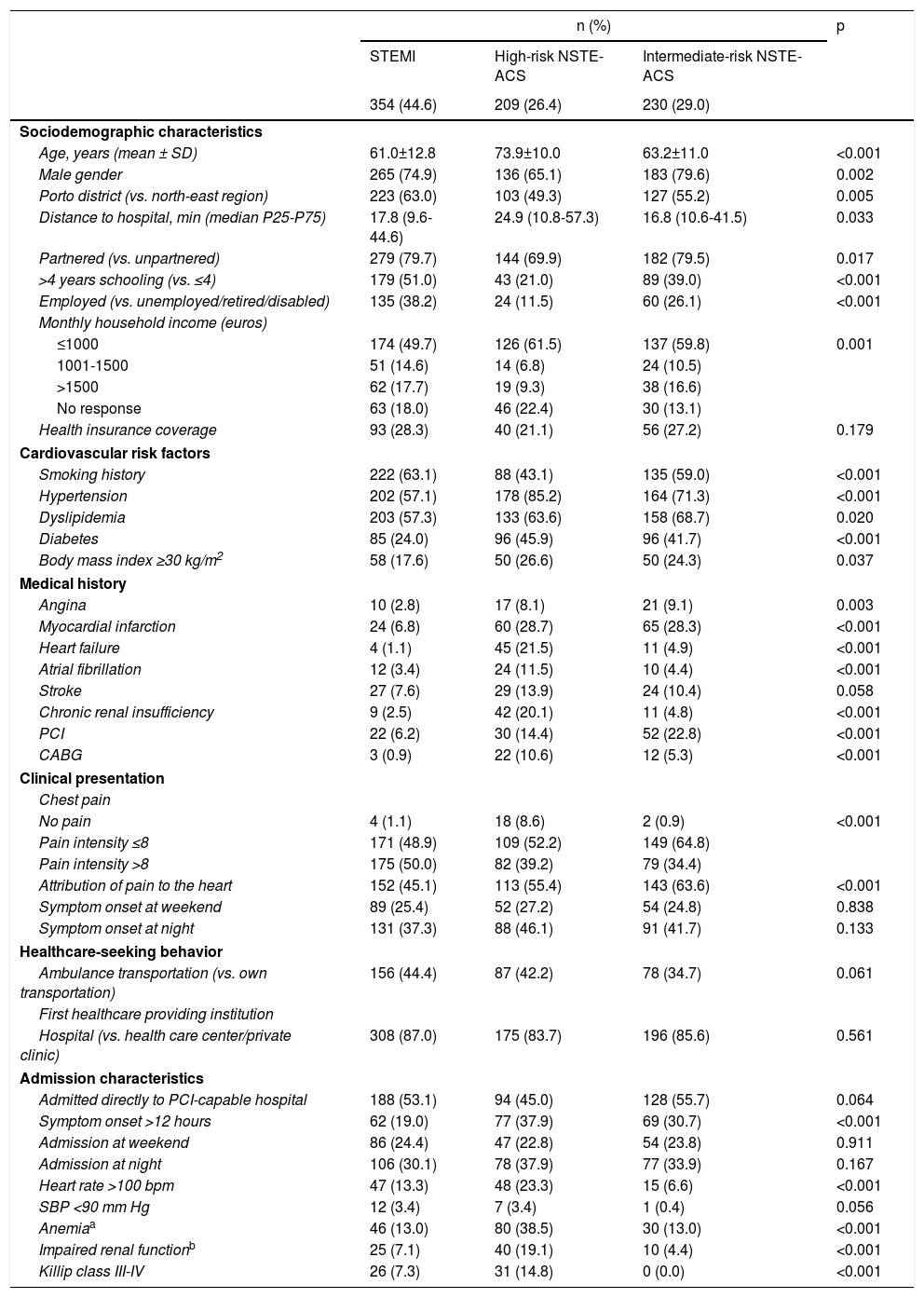
ResultsNSTE-ACS patients were significantly older than those with STEMI (Table 1). High-risk NSTE-ACS patients were less often men, Porto inhabitants, and partnered, were less educated and had lower income. The prevalence of cardiovascular risk factors was greater among NSTE-ACS patients, except for a history of smoking, which was more frequent in those with STEMI. Overall, nearly 60% of the patients used their own means of transportation to seek healthcare services, nearly 15% chose primary healthcare centers as their first healthcare provider, and just over half were admitted directly to a PCI-capable hospital.
Patient characteristics according to diagnosis and risk stratification.
| n (%) | p | |||
|---|---|---|---|---|
| STEMI | High-risk NSTE-ACS | Intermediate-risk NSTE-ACS | ||
| 354 (44.6) | 209 (26.4) | 230 (29.0) | ||
| Sociodemographic characteristics | ||||
| Age, years (mean ± SD) | 61.0±12.8 | 73.9±10.0 | 63.2±11.0 | <0.001 |
| Male gender | 265 (74.9) | 136 (65.1) | 183 (79.6) | 0.002 |
| Porto district (vs. north-east region) | 223 (63.0) | 103 (49.3) | 127 (55.2) | 0.005 |
| Distance to hospital, min (median P25-P75) | 17.8 (9.6-44.6) | 24.9 (10.8-57.3) | 16.8 (10.6-41.5) | 0.033 |
| Partnered (vs. unpartnered) | 279 (79.7) | 144 (69.9) | 182 (79.5) | 0.017 |
| >4 years schooling (vs. ≤4) | 179 (51.0) | 43 (21.0) | 89 (39.0) | <0.001 |
| Employed (vs. unemployed/retired/disabled) | 135 (38.2) | 24 (11.5) | 60 (26.1) | <0.001 |
| Monthly household income (euros) | ||||
| ≤1000 | 174 (49.7) | 126 (61.5) | 137 (59.8) | 0.001 |
| 1001-1500 | 51 (14.6) | 14 (6.8) | 24 (10.5) | |
| >1500 | 62 (17.7) | 19 (9.3) | 38 (16.6) | |
| No response | 63 (18.0) | 46 (22.4) | 30 (13.1) | |
| Health insurance coverage | 93 (28.3) | 40 (21.1) | 56 (27.2) | 0.179 |
| Cardiovascular risk factors | ||||
| Smoking history | 222 (63.1) | 88 (43.1) | 135 (59.0) | <0.001 |
| Hypertension | 202 (57.1) | 178 (85.2) | 164 (71.3) | <0.001 |
| Dyslipidemia | 203 (57.3) | 133 (63.6) | 158 (68.7) | 0.020 |
| Diabetes | 85 (24.0) | 96 (45.9) | 96 (41.7) | <0.001 |
| Body mass index ≥30 kg/m2 | 58 (17.6) | 50 (26.6) | 50 (24.3) | 0.037 |
| Medical history | ||||
| Angina | 10 (2.8) | 17 (8.1) | 21 (9.1) | 0.003 |
| Myocardial infarction | 24 (6.8) | 60 (28.7) | 65 (28.3) | <0.001 |
| Heart failure | 4 (1.1) | 45 (21.5) | 11 (4.9) | <0.001 |
| Atrial fibrillation | 12 (3.4) | 24 (11.5) | 10 (4.4) | <0.001 |
| Stroke | 27 (7.6) | 29 (13.9) | 24 (10.4) | 0.058 |
| Chronic renal insufficiency | 9 (2.5) | 42 (20.1) | 11 (4.8) | <0.001 |
| PCI | 22 (6.2) | 30 (14.4) | 52 (22.8) | <0.001 |
| CABG | 3 (0.9) | 22 (10.6) | 12 (5.3) | <0.001 |
| Clinical presentation | ||||
| Chest pain | ||||
| No pain | 4 (1.1) | 18 (8.6) | 2 (0.9) | <0.001 |
| Pain intensity ≤8 | 171 (48.9) | 109 (52.2) | 149 (64.8) | |
| Pain intensity >8 | 175 (50.0) | 82 (39.2) | 79 (34.4) | |
| Attribution of pain to the heart | 152 (45.1) | 113 (55.4) | 143 (63.6) | <0.001 |
| Symptom onset at weekend | 89 (25.4) | 52 (27.2) | 54 (24.8) | 0.838 |
| Symptom onset at night | 131 (37.3) | 88 (46.1) | 91 (41.7) | 0.133 |
| Healthcare-seeking behavior | ||||
| Ambulance transportation (vs. own transportation) | 156 (44.4) | 87 (42.2) | 78 (34.7) | 0.061 |
| First healthcare providing institution | ||||
| Hospital (vs. health care center/private clinic) | 308 (87.0) | 175 (83.7) | 196 (85.6) | 0.561 |
| Admission characteristics | ||||
| Admitted directly to PCI-capable hospital | 188 (53.1) | 94 (45.0) | 128 (55.7) | 0.064 |
| Symptom onset >12 hours | 62 (19.0) | 77 (37.9) | 69 (30.7) | <0.001 |
| Admission at weekend | 86 (24.4) | 47 (22.8) | 54 (23.8) | 0.911 |
| Admission at night | 106 (30.1) | 78 (37.9) | 77 (33.9) | 0.167 |
| Heart rate >100 bpm | 47 (13.3) | 48 (23.3) | 15 (6.6) | <0.001 |
| SBP <90 mm Hg | 12 (3.4) | 7 (3.4) | 1 (0.4) | 0.056 |
| Anemiaa | 46 (13.0) | 80 (38.5) | 30 (13.0) | <0.001 |
| Impaired renal functionb | 25 (7.1) | 40 (19.1) | 10 (4.4) | <0.001 |
| Killip class III-IV | 26 (7.3) | 31 (14.8) | 0 (0.0) | <0.001 |
CABG: cardiac artery bypass grafting; NSTE-ACS: non-ST-elevation acute coronary syndrome; P25: 25th percentile; P75: 75th percentile; PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction.
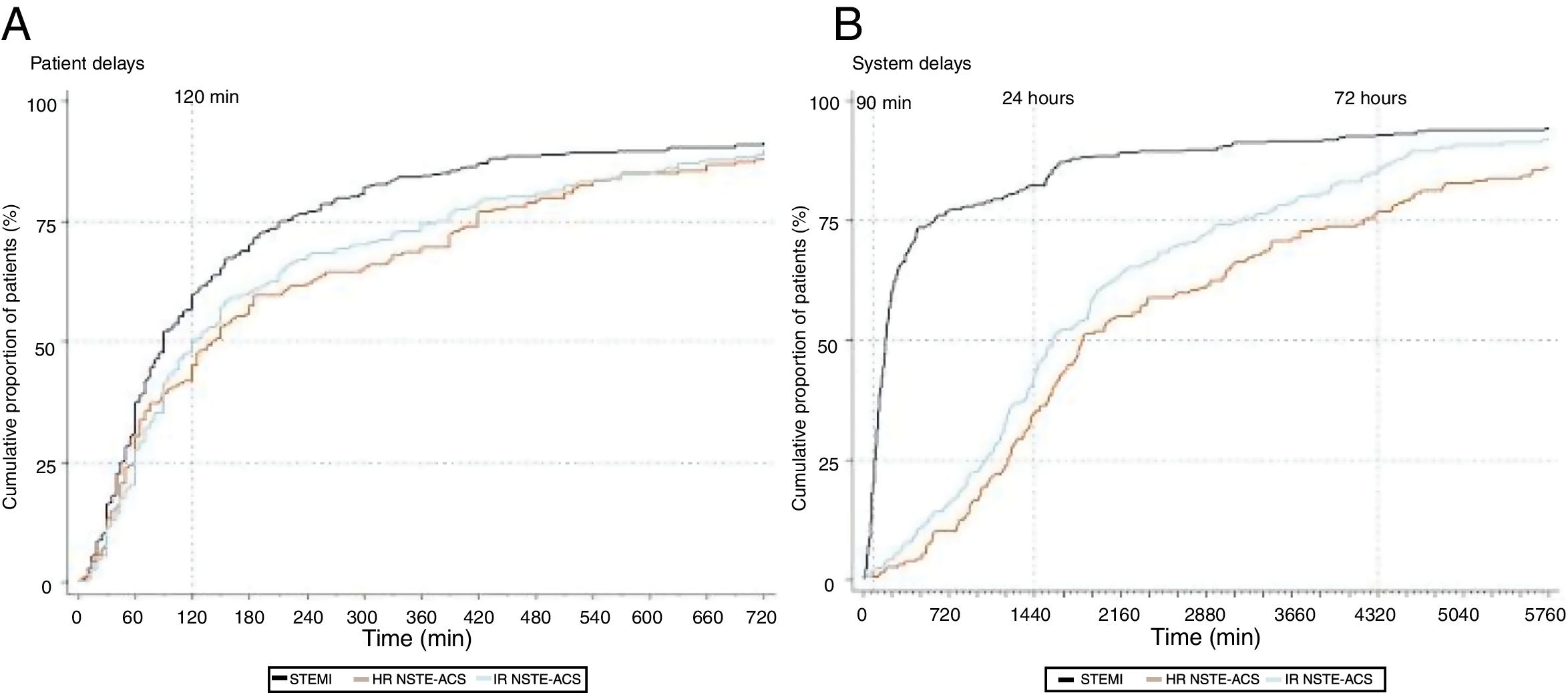
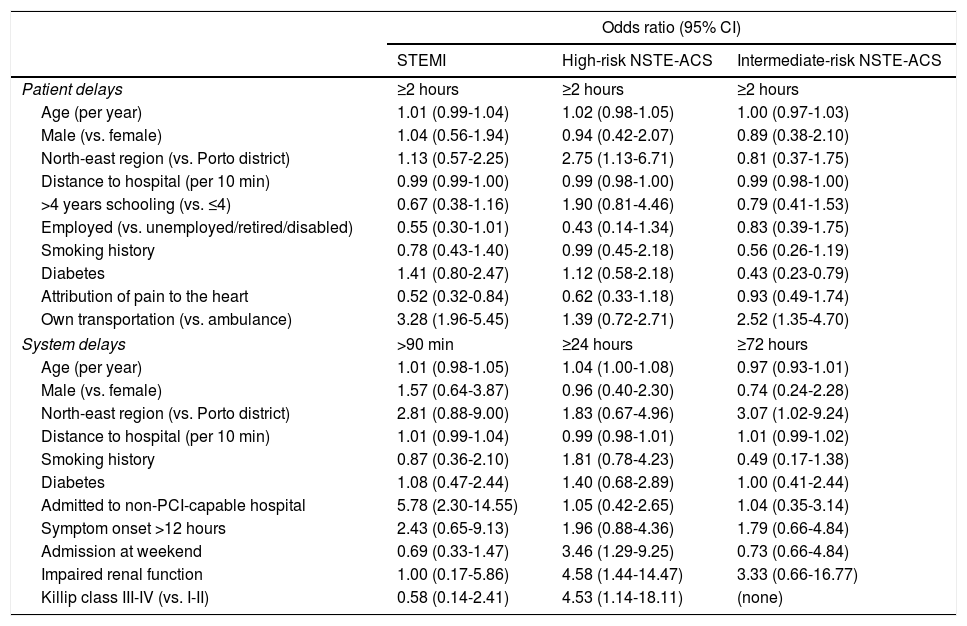
Median time between symptom onset and FMC was 90 min (IQR: 46-210), 140 min (60-420), and 123 min (60-390) for STEMI, high-risk NSTE-ACS, and intermediate-risk NSTE-ACS patients, respectively (Figure 2). Symptom onset-FMC delay greater than 120 min was more frequent in the high-risk NSTE-ACS group (57.7%), followed by intermediate-risk NSTE-ACS (52.1%) and STEMI (43.3%). Patients who correctly attributed their symptoms to the heart were less likely to experience a delay between symptoms and FMC ≥120 min, although the difference was statistically significant only for those with STEMI (Table 2). By contrast, the risk of prolonged delay was greater for patients who used their own transportation, compared with ambulance use. Among high-risk NSTE-ACS, patients from the north-east region had a higher risk of prolonged delay than patients from Porto, whereas among intermediate-risk NSTE-ACS, patients with diabetes had a lower risk of prolonged delay.
Multivariate models of factors associated with prolonged patient and system delays, according to diagnosis and risk stratification.
| Odds ratio (95% CI) | |||
|---|---|---|---|
| STEMI | High-risk NSTE-ACS | Intermediate-risk NSTE-ACS | |
| Patient delays | ≥2 hours | ≥2 hours | ≥2 hours |
| Age (per year) | 1.01 (0.99-1.04) | 1.02 (0.98-1.05) | 1.00 (0.97-1.03) |
| Male (vs. female) | 1.04 (0.56-1.94) | 0.94 (0.42-2.07) | 0.89 (0.38-2.10) |
| North-east region (vs. Porto district) | 1.13 (0.57-2.25) | 2.75 (1.13-6.71) | 0.81 (0.37-1.75) |
| Distance to hospital (per 10 min) | 0.99 (0.99-1.00) | 0.99 (0.98-1.00) | 0.99 (0.98-1.00) |
| >4 years schooling (vs. ≤4) | 0.67 (0.38-1.16) | 1.90 (0.81-4.46) | 0.79 (0.41-1.53) |
| Employed (vs. unemployed/retired/disabled) | 0.55 (0.30-1.01) | 0.43 (0.14-1.34) | 0.83 (0.39-1.75) |
| Smoking history | 0.78 (0.43-1.40) | 0.99 (0.45-2.18) | 0.56 (0.26-1.19) |
| Diabetes | 1.41 (0.80-2.47) | 1.12 (0.58-2.18) | 0.43 (0.23-0.79) |
| Attribution of pain to the heart | 0.52 (0.32-0.84) | 0.62 (0.33-1.18) | 0.93 (0.49-1.74) |
| Own transportation (vs. ambulance) | 3.28 (1.96-5.45) | 1.39 (0.72-2.71) | 2.52 (1.35-4.70) |
| System delays | >90 min | ≥24 hours | ≥72 hours |
| Age (per year) | 1.01 (0.98-1.05) | 1.04 (1.00-1.08) | 0.97 (0.93-1.01) |
| Male (vs. female) | 1.57 (0.64-3.87) | 0.96 (0.40-2.30) | 0.74 (0.24-2.28) |
| North-east region (vs. Porto district) | 2.81 (0.88-9.00) | 1.83 (0.67-4.96) | 3.07 (1.02-9.24) |
| Distance to hospital (per 10 min) | 1.01 (0.99-1.04) | 0.99 (0.98-1.01) | 1.01 (0.99-1.02) |
| Smoking history | 0.87 (0.36-2.10) | 1.81 (0.78-4.23) | 0.49 (0.17-1.38) |
| Diabetes | 1.08 (0.47-2.44) | 1.40 (0.68-2.89) | 1.00 (0.41-2.44) |
| Admitted to non-PCI-capable hospital | 5.78 (2.30-14.55) | 1.05 (0.42-2.65) | 1.04 (0.35-3.14) |
| Symptom onset >12 hours | 2.43 (0.65-9.13) | 1.96 (0.88-4.36) | 1.79 (0.66-4.84) |
| Admission at weekend | 0.69 (0.33-1.47) | 3.46 (1.29-9.25) | 0.73 (0.66-4.84) |
| Impaired renal function | 1.00 (0.17-5.86) | 4.58 (1.44-14.47) | 3.33 (0.66-16.77) |
| Killip class III-IV (vs. I-II) | 0.58 (0.14-2.41) | 4.53 (1.14-18.11) | (none) |
CI: confidence interval; NSTE-ACS: non-ST-elevation acute coronary syndrome; PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction.
Regarding system delay, median time between FMC and PCI was 177 min (95-435) for STEMI patients, and 78.0% experienced a delay exceeding 90 min (Figure 2). Median time between FMC and angiography was 30.9 hours (20.3-70.2) for high-risk NSTE-ACS and 26.6 hours (17.1-52.0) for intermediate-risk NSTE-ACS; the delay exceeded the recommended timeframes in 65.8% of patients with high-risk NSTE-ACS and in 14.2% of those with intermediate-risk NSTE-ACS. Patients living in the north-east region were more likely to be treated beyond the recommended timeframe, although the differences were not statistically significant in all groups (Table 2). The risk of delay >90 min was nearly six-fold higher for patients with STEMI admitted to non-PCI-capable hospitals, while for high-risk NSTE-ACS, patients with hospital admission during the weekend, impaired renal failure, and in Killip class III-IV were more likely to have delays ≥24 hours between FMC and angiography.
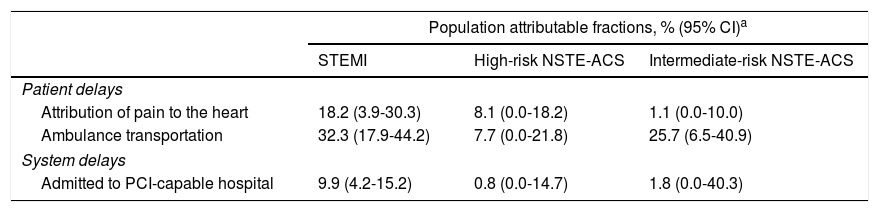
The proportion of patients with delays between symptom onset and FMC >120 min could have been reduced by nearly 50% for STEMI and 25% for intermediate-risk NSTE-ACS if all patients had correctly recognized their symptoms and had been transported by ambulance (Table 3). Further, the proportion of STEMI patients with delays beyond the recommended timeframe could be reduced by around 10% if all patients were admitted directly to a PCI-capable hospital.
Impact of modifiable determinants on patient and system delays, according to diagnosis and risk stratification.
| Population attributable fractions, % (95% CI)a | |||
|---|---|---|---|
| STEMI | High-risk NSTE-ACS | Intermediate-risk NSTE-ACS | |
| Patient delays | |||
| Attribution of pain to the heart | 18.2 (3.9-30.3) | 8.1 (0.0-18.2) | 1.1 (0.0-10.0) |
| Ambulance transportation | 32.3 (17.9-44.2) | 7.7 (0.0-21.8) | 25.7 (6.5-40.9) |
| System delays | |||
| Admitted to PCI-capable hospital | 9.9 (4.2-15.2) | 0.8 (0.0-14.7) | 1.8 (0.0-40.3) |
CI: confidence interval; NSTE-ACS: non-ST-elevation acute coronary syndrome; PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction.
Despite the recognized benefits of timely treatment for ACS and substantial investment in this area, a large proportion of patients still fail to receive treatment within recommended timeframes. Nearly one-half of the patients in our study experienced delays ≥120 min from symptom onset to FMC. The use of prehospital ambulances and the correct interpretation of symptoms, both of which can be improved by educational interventions, were found to play a key role in patient delays. Moreover, system delays exceeded the recommendations for 78% of STEMI patients and 66% of higher-risk NSTE-ACS patients. Admission to a PCI-capable hospital was associated with shorter delays for patients with STEMI, whereas admission during weekends and complications at admission were associated with prolonged system delays for patients with high-risk NSTE-ACS.
The implementation of various American (D2B Alliance, Mission: Lifeline) and European (Stent for Life) initiatives has aimed to increase the proportion of STEMI patients with timely access to primary PCI.10,11 In Portugal the Stent for Life initiative was implemented in 2011, however, four years later, no significant differences were observed in symptom onset-FMC time (114 min in 2011 vs. 119 min in 2015).12,13 Median STEMI patient delays in our study are lower, but nearly half of patients continue to experience delays of ≥120 min from symptom onset to FMC. Several recent European studies have documented similar proportions, in some cases unchanged over time.14,15
Studies on delays specifically among NSTE-ACS patients are scarce and analyze only prehospital delays (time between symptom onset and hospital presentation), rather than patient delays.16–20 These studies, although not directly comparable to ours, identify patient delay as a major contributor to prehospital delay. Similarly to our results, in the CRUSADE study, which analyzed 104 622 NSTE-ACS patients, median time from symptom onset to hospital presentation was 156 min and the delay was ≥120 min for 59% of the patients.16
Additionally, our findings suggest that patients with STEMI sought treatment earlier than patients with NSTE-ACS, in line with previous reports.17–19 Since patients typically cannot identify the type of ACS they are experiencing, there may be a link between pathophysiological processes and symptom severity that expedites STEMI patients’ decision to seek healthcare services; in our study patients with STEMI reported higher pain intensity. The differences in patient delays may also be explained by the different characteristics of NSTE-ACS and STEMI patients. In comparison with STEMI patients, NSTE-ACS patients were more frequently women, older, and had a higher prevalence of chronic health conditions. Such patients are more likely to experience atypical symptoms,21 which may be associated with longer delays.
Regardless of diagnosis and risk stratification, the correct interpretation of symptoms and the use of ambulances were associated with shorter patient delays. These factors have previously been found to be associated with timely access to reperfusion among STEMI patients.12,13,22,23 Furthermore, patients who recognize their symptoms as cardiac were more likely to use emergency ambulances,24 which can result in a 30-minute reduction in delay from symptom onset to presentation.22 Our results show, however, that nearly one-half of patients did not attribute their symptoms to the heart, nor did they use prehospital ambulances. Therefore, considerably more effort could be put into educating communities, as well as individuals, about symptom awareness and efficient healthcare-seeking behavior.25 We estimate that the proportion of patients with delays of ≥2 hours could be reduced by nearly 50% if all patients correctly attributed their symptoms to the heart and used ambulances to reach healthcare facilities.
The proportion of patients with STEMI who, due to system delays, fail to receive treatment as recommended remains high. System delays exceeded 90 min in approximately 75% of STEMI patients, which echoes previously published studies in Portugal.13,26 Similar results from Finland suggest there have been no substantial improvements in system delays over time, as the percentage of patients with treatment delays exceeding 90 min only decreased from 77% in 2007-2008 to 75% in 2011-2012.15 The median system delay in our study coincided with the upper limit of the range (60-177 min) reported in a European study on 30 countries.27 These findings call for more effort in addressing modifiable healthcare system factors that may explain this delay.
The healthcare system is failing to manage STEMI patients with early invasive strategies, but its performance has affected NSTE-ACS patients as well, since two-thirds of patients with high-risk NSTE-ACS were treated beyond 24 hours. Data from the Portuguese Registry on Acute Coronary Syndromes show that, between 2002 and 2015, there were significant increases in the proportion of patients who underwent coronary angiography within 24 hours.28 However, in 2015 still one-half of patients were treated beyond the recommended time limit,28 which is similar with the proportion reported in a recent study from the SWEDEHEART registry.29 These results support the notion that using existing tools is a key priority to improve risk stratification and adherence to early invasive strategies for higher-risk patients.
Transfer from a non-PCI-capable hospital was the strongest predictor of delay >90 min among STEMI patients, a finding which is in line with previous studies.30,31 Furthermore, patients from the north-east region experienced prolonged system delays, which may be explained by the long transfer distances in rural areas. Although the region itself was not an independent predictor of delays, patients living in rural areas were more likely to be referred to a non-PCI-capable hospital, leading to longer delays.32 In our study, almost half of the patients from the north-east region lived more than 60 min away from the nearest PCI-capable hospital, while such patients accounted only for a very small proportion in the Porto district. Several studies have reported that direct referral to PCI-capable hospitals, along with prehospital electrocardiograms, enables STEMI patients from rural populations to have timely access to PCI.33,34 In this context, the heterogeneous geographic distribution of healthcare resources in Portugal may require adjustments in the healthcare system in order to ensure more equitable access to treatment for ACS, particularly in rural areas. Additionally, high-risk NSTE-ACS patients whose symptoms appeared during the weekend were more likely to experience prolonged system delays. This may be partially explained by the time that the hospitals needed to assemble on-call staff and activate the cardiac catheterization laboratory during off-hours. Finally, admission characteristics related to impaired renal function and Killip class were found to be associated with prolonged system delays for NSTE-ACS patients. These findings may be explained by such patients’ need for more complex stabilization in the acute care phase.30
Study limitationsThis study offers valuable new insights into predictors of both patient and healthcare system delays affecting ACS patients, thus contributing to a better understanding of variations in treatment. Despite its methodological strengths, including a high participation rate and detailed prospective patient characterization collected by trained staff, certain limitations of the study must be acknowledged. First, patients who died before arriving at the hospital or in the hospital emergency department, and patients admitted to other hospital departments, were not included in the study. As these patients are expected to have experienced longer delays, our results might underestimate time delays. Second, although data were collected within 48 hours of admission, we cannot rule out that recall bias might have occurred in some patients. Lastly, our results may not be generalizable to other regions in the country, although the study was conducted in two different settings, one mainly coastal and urban and the other mainly rural and inland, which enabled the comparison of relevant factors regarding access to health care.
ConclusionDue to both patient and system delays, a large proportion of STEMI and high-risk NSTE-ACS patients still fail to have access to timely reperfusion. To improve implementation of the guidelines, symptom awareness and the use of prehospital ambulances should be improved. In addition, the healthcare system should ensure that patients in all geographic locations receive timely reperfusion and that STEMI patients have direct access to PCI-capable hospitals, while also addressing the variation in care during weekends.
Funding sourcesThis study was supported by the European Regional Development Fund (ERDF), through the Operational Programme “Competitiveness and Internationalization”, and national funding from the Foundation for Science and Technology (FCT), Portuguese Ministry of Education and Science (FCOMP-01-0124-FEDER-028709), under the project “Inequalities in coronary heart disease management and outcomes in Portugal” (FCT PTDC/DTP-EPI/0434/2012) and the Unidade de Investigação em Epidemiologia – Instituto de Saúde Pública da Universidade do Porto (EPIUnit) (POCI-01-0145-FEDER-006862; Ref. UID/DTP/04750/2013).
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors are grateful to Christina Yantsides for her review and editing of the manuscript.