Mitral valve aneurysms are rare and occur most commonly in association with aortic valve endocarditis. Transesophageal echocardiography is the most sensitive imaging modality for the diagnosis of this entity and its potential complications, such as leaflet rupture and mitral regurgitation, which mandate prompt surgical intervention.
We present the case of a 70-year-old male patient with aortic valve endocarditis complicated with a ruptured aneurysm of the anterior mitral valve leaflet and associated severe mitral regurgitation, diagnosed by transesophageal echocardiography, with impressive images. We hypothesized that the aneurysm developed through direct extension of infection from the aortic valve or from a prolapsing aortic vegetation, with abscess formation and subsequent rupture and drainage. This case highlights the importance of appropriate imaging for early detection and timely surgical intervention (repair or replacement) to prevent fatal outcomes.
Os aneurismas da válvula mitral são raros e, habitualmente, encontram-se associados a endocardite da válvula aórtica. O ecocardiograma transesofágico é a modalidade imagiológica mais sensível para o diagnóstico desta entidade e das suas potenciais complicações, tais como perfuração e regurgitação mitral, que exigem intervenção cirúrgica urgente.
Apresentamos o caso clínico de um doente de 70 anos, do sexo masculino, com endocardite da válvula aórtica complicada por aneurisma perfurado do folheto anterior da válvula mitral e regurgitação mitral grave associada, diagnosticada por ecocardiograma transesofágico com imagens muito ilustrativas. Admitimos como hipótese, mais provável, que o aneurisma se desenvolveu por extensão direta da infeção a partir da válvula aórtica ou do prolapso de uma vegetação aórtica, com formação de abcesso e subsequente rotura e drenagem. Este caso realça a importância das modalidades imagiológicas adequadas no diagnóstico precoce do aneurisma da válvula mitral e da intervenção cirúrgica atempada (plastia ou substituição) na prevenção de eventos fatais.
A 70-year-old male patient with type 2 diabetes mellitus and chronic hepatitis C virus infection presented to the emergency department with a four-month history of intermittent fever, night sweats, weight loss and progressive dyspnea. There was no past history of cardiovascular disease or illicit drug abuse. On physical examination, he was febrile (38.1°C), his pulse was 124 bpm, blood pressure was 114/73 mmHg and he had signs of left heart failure. Cardiac auscultation disclosed a grade 3/6 pansystolic murmur at the apex. Blood cultures were positive for Streptococcus viridans.
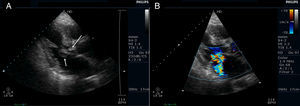
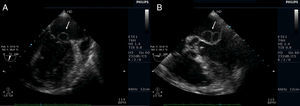
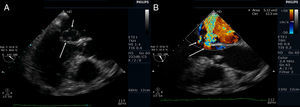
Transthoracic echocardiography (TTE) showed multiple aortic valve vegetations (some prolapsing into the left ventricular outflow tract), mild aortic regurgitation and a large saccular bulge originating from the anterior mitral valve leaflet and protruding into the left atrium with severe mitral regurgitation (Figure 1); left ventricular systolic function was normal. With transesophageal echocardiography (TEE) it became clear that the saccular bulging on the mitral valve was an anterior mitral valve leaflet aneurysm with two rupture sites (Figure 2). Color flow Doppler showed two regurgitant jets: one through the coaptation margin of the mitral valve leaflets and the other across the perforated aneurysm (Figure 3). There was no evidence of either aortic ring abscess formation or extension along the mitral-aortic intervalvular fibrosa.
Transthoracic echocardiogram (parasternal long-axis view). (A) Saccular bulge on the atrial side of the anterior mitral valve leaflet (small arrow) and a vegetation on the aortic valve prolapsing into the left ventricular outflow tract, in close contact with the anterior mitral valve leaflet (long arrow); (B) significant mitral regurgitation (color flow Doppler).
Transesophageal echocardiogram (3-chamber view). (A) Ruptured aneurysm of the anterior mitral valve leaflet. The long arrow points to the entry site and the small arrow points to the exit site of the aneurysm; (B) color Doppler image showing two mitral regurgitation jets: a small jet through the leaflet coaptation point (small arrow) and a much more significant one through the aneurysm (long arrow).
Antibiotic therapy was started with vancomycin plus gentamicin (due to penicillin allergy) for infective endocarditis (IE). After clinical stabilization, he was referred for urgent cardiac surgery. Given his clinical stability, surgery was delayed for four more days of antibiotic therapy. The patient died suddenly one day before scheduled surgical intervention.
DiscussionMitral valve aneurysms (MVAs) are uncommon but potentially serious complications, occurring most frequently in association with aortic valve endocarditis.1–4 The first case was reported by Morand in 1729.5 In 1970, Gonzalez-Lavin et al.6 reported secondary involvement of the mitral valve with leaflet aneurysm in two (3.4%) patients out of 58 who underwent aortic valve surgery for IE. Similar results were reported in 1992 by Karalis et al.7 in a group of 55 patients with aortic valve endocarditis evaluated by TEE. More recent reports present a very low incidence, around 0.2-0.29% of patients who undergo TEE.2,4
Although most frequently associated with IE, other non-infectious etiologies have been reported including congenital cardiac structural defects, extreme forms of mitral valve prolapse, Libman-Sacks endocarditis and connective tissue disorders such as Marfan syndrome, Ehlers-Danlos syndrome, pseudoxanthoma elasticum and osteogenesis imperfecta.4,9–13
Because MVA is rare in the absence of IE, an infectious etiology will be at least partly responsible for leaflet degeneration. Proposed mechanisms for the development of MVA in the setting of aortic valve endocarditis include direct extension of the infection through the mitral-aortic intervalvular fibrosa to the anterior mitral valve leaflet with abscess formation and subsequent drainage, seeding of the anterior mitral valve leaflet by the aortic regurgitant jet (‘jet lesion’),1,3,8,14–16 or through direct contact with an aortic vegetation prolapsing into the left ventricular outflow tract (‘kissing vegetation’), especially if the vegetation exceeds 6 mm in size.17 These processes are thought to cause localized inflammation, weakness, dissection, and subsequent expansion of the valvular tissue, which in turn protrudes toward the left atrium due to ventricular systolic pressure, leading to aneurysm formation.2,18–21 In our case, because aortic regurgitation was only mild, we believe that the most likely mechanism for aneurysm development was previous abscess formation (secondary to direct extension of infection from the aortic valve or a prolapsing aortic vegetation) followed by rupture and drainage.
The diagnosis may be suspected by TTE but TEE is more sensitive for detecting MVA, leaflet rupture and mitral regurgitation.2,4,22–25 Recently, a few reports have highlighted the role of real-time three-dimensional echocardiography to better define the anatomical features of MVA preoperatively.16,26–28
Echocardiography shows MVA as a localized saccular bulge of the mitral valve leaflet toward the left atrium communicating with the left ventricle, expanding in systole and collapsing in diastole.2 Diastolic expansion can occur in cases of MVA rupture or severe aortic regurgitation.28 These lesions can vary from a few mm to 4 cm in diameter and may contain thrombi.8 The anterior leaflet is much more commonly involved than the posterior leaflet.2–4 The diagnosis of perforation is based on the presence of a high-velocity regurgitant jet traversing the aneurysm in systole and further supported by demonstration of an interrupted segment of the aneurysm by two-dimensional examination.4,29
MVA must be differentiated from various abnormalities with similar echocardiographic appearance including severe mitral valve prolapse, diverticulum of the mitral valve, large valvular vegetation, flail valve leaflet, blood cyst of the papillary muscle, cystic atrial myxoma or non-endothelialized cyst of the mitral valve.24,30 Color flow Doppler helps distinguish an aneurysm from other similar-looking abnormalities by demonstrating direct communication between the aneurysm and the left ventricle.22
Potential complications of these aneurysms include rupture, thromboembolism and recurrent infection. MVAs can also cause severe mitral regurgitation due to perforation of the aneurysm or as a result of leaflet coaptation defect caused by the mass effect of the aneurysm, and may precipitate rapid deterioration of hemodynamic status.
The optimal management strategy for MVAs has not yet been well defined. A few reports have shown that conservative management of uncomplicated MVAs, with close follow-up, is possible.2,31 In cases of MVA rupture or severe mitral regurgitation or if the patient needs aortic valve replacement surgery, then mitral valve surgery should be considered.4,15,31 Conservative mitral valve surgery for aneurysm correction is not always possible; it depends on the degree of valve destruction and the anatomic disorder.32 When it is unsuitable for repair,32–34 mitral valve replacement may be the only viable option.25,35,36
In conclusion, MVAs are rare but potentially life-threatening complications and should be carefully considered in the evaluation of every patient with aortic valve endocarditis. We report a case with impressive images of aortic valve endocarditis complicated by perforated mitral valve aneurysm and severe mitral regurgitation, diagnosed by TEE. This case highlights the importance of early diagnosis with appropriate imaging and timely surgical intervention to prevent fatal outcomes.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors wish to thank the Cardiothoracic Surgery Department of Centro Hospitalar S. João, Porto, for rapidly accepting the patient transfer.