Inadvertent endocardial placement of a pacing lead in the left ventricle through the aortic valve is a rare complication with an unknown incidence because of inadequate reporting. Reported cases are usually the result of lead insertion via the subclavian artery. A possible but very unusual situation is endocardial lead insertion in the left ventricle after aortic arch perforation. We report the case of a 72-year-old woman in whom a screw-in pacing lead accidentally perforated the aortic arch and continued its way through the ascending aorta, aortic valve and left ventricle, after insertion through the left subclavian vein. We describe how this complication was diagnosed, the predisposing factors, the risks it carries and the ways in which devastating consequences have so far been avoided, as the patient refused any surgical intervention including lead removal.
A colocação inadvertida de um elétrodo de pacing ao nível do endocárdio do ventrículo esquerdo, através da válvula aórtica, é uma complicação rara, com uma incidência desconhecida por ser incorretamente relatada. Os casos relatados são, geralmente, o resultado da inserção do elétrodo através da artéria subclávia. Uma situação possível, mas muito incomum, é a colocação do elétrodo ao nível do endocárdio do ventrículo esquerdo, após a perfuração do arco aórtico. Relatamos aqui o caso de uma doente com 72 anos, em que elétrodo do tipo de fixação ativa perfurou acidentalmente o arco aórtico, continuando o seu trajeto através da aorta ascendente, da válvula aórtica e do ventrículo esquerdo, após inserção pela veia subclávia esquerda. Descrevemos a maneira como esta complicação foi diagnosticada, os fatores predisponentes, os riscos associados a essa complicação e as formas possíveis de evitar algumas consequências devastadoras até hoje, considerando que a doente recusou qualquer intervenção cirúrgica, incluindo a extração do elétrodo.
Inadvertent placement of the pacing lead in the left ventricle (LV) through the aortic valve is a rare complication of artificial pacemaker implantation with an unknown incidence because of inadequate reporting. Reported cases are usually the result of inadvertent subclavian artery puncture.1–5 Regarding insertion of the lead through the aorta after aortic wall perforation, to the best of our knowledge there are only two cases reported in the literature.6,7 In both of them the lead was removed, and there is no evidence in the literature about possible predisposing factors or the management of such cases when lead removal is unfeasible.
Case reportA 72-year-old woman, particularly short, obese and displaying kyphosis, with moderate aortic regurgitation, severe mitral regurgitation, and an ascending aortic aneurysm, underwent implantation of a single-chamber pacemaker for severely symptomatic complete atrioventricular block and atrial fibrillation. During the intervention, because the cephalic vein was inaccessible, the left subclavian vein was punctured (with evidence of venous blood) and a Biotronik Selox ST 60 screw-in pacing lead was placed in an endocavitary position using a 7 F introducer, with good pacing and sensing thresholds (0.5 V and 8.3 mV, respectively). The procedure, performed by an experienced operator, was uneventful.
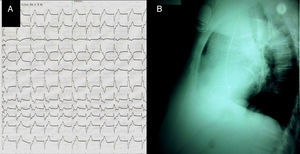
After the intervention, two unusual findings were made: a right bundle branch block (RBBB) pattern of the paced rhythm (with electrical axis of −150°, precordial transition in V4, rS in DI, RsR’ in V1–V2) (Figure 1A); and a posterior lead orientation on the lateral chest radiograph (Figure 1B). We suspected the lead to be in a branch of the coronary sinus. The uncertainty prompted further investigation. Transthoracic echocardiography (Figure 2A) followed by transesophageal examination revealed the pacing lead entering the LV through the aortic valve and inserted in the mid part of the lateral wall.
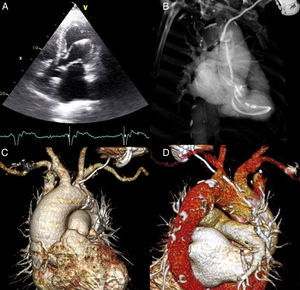
(A) Transthoracic echocardiography, apical 4-chamber view, showing the pacing lead passing through the aortic valve to insert in the left ventricular lateral wall; (B) thoracic computed tomography angiography (CTA), maximum intensity projection image, showing the entire trajectory of the pacing lead through the aneurysmal ascending aorta, aortic valve and left ventricle; (C) volume-rendered thoracic CTA showing pacing lead perforating the atheromatous aortic arch; (D) volume-rendered thoracic CTA showing perforation site between the left common carotid artery and the brachiocephalic trunk.
Thoracic computed tomography (CT) angiography highlighted the entire trajectory; it revealed the lead leaving the subclavian vein, penetrating the aortic wall between the brachiocephalic trunk and the emergence of the left common carotid artery, and continuing its way to the left ventricle through the ascending aorta and aortic valve (Figure 2B–D). The ascending aortic aneurysm (5.8 cm×5.7 cm), with calcified atherosclerotic plaques, was confirmed without signs of dissection or hematoma.
The patient showed no clinical manifestation of this complication and refused any surgical intervention, including lead removal. She remained on heart failure medication and oral anticoagulation. No clinical or echocardiographic worsening related to the lead's position occurred during 12 months of follow-up. Chronic sensing and pacing parameters remained within normal ranges.
DiscussionInadvertent endocardial LV placement of a pacing lead by aortic perforation is both possible and easy to overlook if the electrocardiographic and radiologic signs are misinterpreted. The interventional cardiologist must be aware of the situation as further unprotected interventional lead manipulation or extraction could result in serious mechanical complications. An RBBB pattern of the paced rhythm may be encountered in both LV and right ventricular pacing. In order to assess the lead's position, Okmen et al. proposed electrocardiographic criteria (left axis deviation between −30° and −90°, precordial transition in V3, absence of S wave in DI and qR or RS pattern in V1) that proved to be highly sensitive and specific for true right ventricular pacing; not meeting these criteria, as in our case, strongly suggests a pacing site outside the right ventricle.8
Aortic perforation by a pacing lead can be a life-threatening event; it carries a high risk of systemic thromboembolism due to the LV endocardial lead position in the systemic circulation and, in this case, to atrial fibrillation,3,9 and mechanical complications (aortic wall trauma resulting in massive hemorrhage, or trauma to the lead, aortic valve or coronary arteries).1,3 In our patient, appropriate oral anticoagulation (INR between 2 and 3) prevented thromboembolic events, while calcification and stiffness of the aortic wall possibly prevented aortic laceration and internal bleeding at the time of perforation. The coronary arteries were spared as the lead entered the left ventricle directly without engaging the coronary ostia. No trauma to the aortic valve or worsening of regurgitation was detected by echocardiography but these possible negative effects remain to be further assessed.
In both previously published reports, inadvertent placement of the pacing lead in the left ventricle after perforation of the aortic arch was symptomatic (recurrent left laryngeal nerve irritation and lead thrombosis,6 and large hemothorax and myocardial infarction7). One of the patients had a persistent left superior vena cava with an absent brachiocephalic vein that might have facilitated the complication.6 The leads were removed by conventional surgery through a median sternotomy with direct surgical closure of the aorta,6 and percutaneously by concomitant endovascular stent grafting of the aorta,7 respectively. In our patient the unusual position of normal anatomical structures facilitated this complication. Venous blood detected when puncturing, and the radiologic appearance of the aortic aneurysm, created the impression of a normal trajectory of the guide wire and lead (subclavian vein-superior vena cava-right heart), masking the complication during the intervention. At the time of the diagnosis, percutaneous removal was considered to carry a significant risk of mechanical complications. The recommended treatment for symptomatic malpositioned leads in the left ventricle consists of surgical removal,7,9 but as the patient was asymptomatic and refused any surgical intervention, we had no choice but to leave the lead in place. We chose lifelong oral anticoagulation because, besides the indication for the patient's atrial fibrillation, it has proved successful in asymptomatic patients with the lead inserted in the LV (usually through an atrial septal defect) and when lead removal was impossible.4,9
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
This work was accomplished under the framework of the European Social Fund, Human Resources Development Operational Programme 2007–2013, project no. POSDRU/159/1.5/S/136893.