Young athletes are considered the healthiest group in society. Although rare, there are still reports of sudden death or cardiac arrest on the playing fields. Clinical evaluation is of paramount importance for the identification of possible pathological states that confer increased risk of these events. Interpretation of the electrocardiogram of young athletes can help identify changes associated with heart disease that might preclude the participation in sports. In this context, it is essential to recognize the electrocardiographic patterns that represent the structural and electrical remodeling resulting from continued adaptation to exercise, and which thus do not increase the risk of adverse events during exercise. The European Society of Cardiology (ESC) and the American Heart Association (AHA) have issued consensus documents summarizing which electrocardiographic abnormalities should be considered ‘physiological’, resulting from adaptation to exercise (‘athlete's heart’), and which should be considered pathological and thus require further study. However, the two societies have different approaches with respect to the electrocardiographic screening of athletes. This paper provides a brief review of current evidence regarding the electrocardiographic findings considered normal and abnormal in athletes, and presents the arguments of the ESC and AHA for electrocardiographic screening in this population.
Os jovens atletas são considerados como o grupo mais saudável da sociedade. Apesar de raros, surgem ainda relatos de morte súbita ou paragem cardiorrespiratória nos campos de jogo. A avaliação clínica reveste-se de primordial importância para a identificação de eventuais estados patológicos que confiram risco acrescido destes eventos. A interpretação do eletrocardiograma destes jovens atletas pode permitir identificar alterações associadas a patologia cardíaca que condicione ou não a prática do exercício físico. Neste contexto, torna-se essencial reconhecer os padrões eletrocardiográficos que representam a remodelagem estrutural e elétrica resultantes da adaptação ao exercício continuado, e que por sua vez não acarretam risco acrescido de eventos adversos durante a prática de exercício. As Sociedades Europeia e Americana de Cardiologia emitiram documentos de consenso que procuram resumir quais as alterações eletrocardiográficas consideradas como «fisiológicas» e resultantes da adaptação ao exercício (coração de atleta) e quais as alterações consideradas como patológicas e que portanto implicam o estudo subsequente dos casos em que são identificadas. Estas sociedades apresentam, no entanto uma abordagem diferente no que se refere ao rastreio eletrocardiográfico de atletas. Este artigo fornece uma breve revisão da evidência atual referente aos achados eletrocardiográficos considerados como normais e anormais em atletas, assim como apresenta as argumentações das Sociedades Europeia e Americana de Cardiologia referentes ao rastreio eletrocardiográfico nesta população.
“Mens sana in corpore sano” (“a sound mind in a healthy body”) is an aphorism known by millions worldwide. Sudden death is a tragic event on any occasion. However, if it occurs in an otherwise healthy, highly trained athlete, the impact is greater, because athletes are regarded as the healthiest group in society. Sudden death during sports is an extremely rare event, but has been described since ancient Greece: Pheidippides (490 BC) ran from Marathon to Athens to announce the Greek victory over the Persians, and after delivering the message he collapsed and died.
Interpretation of the athlete's electrocardiogram (ECG) can reveal changes associated with cardiac disease. However, ECG changes in athletes are common and usually reflect structural and electrical remodeling of the heart as an adaptation to regular physical training (known as athlete's heart). On the other hand, abnormalities on a young athlete's ECG can also be an expression of an underlying disease that may carry a risk of sudden cardiac death (SCD) during sports. Lack of knowledge of the ‘normal’ ECG changes in athletes may lead to a diagnostic odyssey with an unfavorable cost–benefit ratio, and often limits participation in sports to athletes whose ECG patterns are within the normal pattern. Many of the ECG changes seen in athletes may overlap with patterns considered pathological in other subgroups of individuals, and this is one of the main limitations of ECG screening in this population – the high number of false positives. Alternatively, signs of potentially lethal cardiovascular disorders may be misinterpreted as normal variants of an athlete's ECG.1
Current European Society of Cardiology (ESC) guidelines include an ECG, besides physical examination, as a prescreening test for athletes. However, concern has focused in particular on the availability of practitioners qualified to interpret ECGs and the burden of false-positive results. The American Heart Association (AHA) and the ESC have both published recommendations for the interpretation of the ECG in athletes.2,3 The present review presents a summary of the most frequent ECG changes considered ‘normal’ in athletes, as well as a brief discussion regarding the advantages and disadvantages of ECG screening in this population.
Cardiac remodeling in athletesAthlete's heart is currently defined as a non-pathological condition in which the heart undergoes morphological and functional changes that result from a process of adaptation to intensive exercise. The heart is usually enlarged and the resting heart rate is lower than normal. Henschen first described the athlete's heart in 1899; using only careful thoracic percussion, he recognized an enlargement of the heart caused by athletic activity in cross-country skiers.4 He concluded that both dilation and hypertrophy were present, involving both sides of the heart, and that these changes were adaptive and improved performance. Since then, numerous descriptions and characterization of the athlete's heart have been performed with the improvements in cardiac imaging such as chest X-ray, echocardiography and cardiac magnetic resonance imaging (CMRI).
The cardiovascular adaptation to exercise differs with the type of conditioning: endurance (dynamic/aerobic) and strength training (isometric/anaerobic). Some sports, such as cycling and rowing, combine both types of conditioning. The adaptation to exercise has two distinct phases: an acute and a chronic phase. The acute response varies according to the type of exercise. In endurance training there is a substantial increase in maximum oxygen uptake, cardiac output, stroke volume and systolic blood pressure, associated with decreased peripheral vascular resistance. On the other hand, strength training causes only a mild increase in maximum oxygen uptake and cardiac output but substantial increases in blood pressure, peripheral vascular resistance and heart rate. Over the long term, the cardiovascular adaptation to aerobic exercise includes increased maximum oxygen uptake (due to increased cardiac output) and an increase in arteriovenous oxygen difference, while strength exercise results in little or no increase in oxygen uptake. Thus, endurance exercise predominantly produces volume load in the left ventricle and strength training mainly causes pressure load.5
Cardiac remodeling secondary systematic training (endurance or strength) is not uniform. In fact, only 50% of trained athletes actually show evidence of cardiac remodeling (change in atrial and ventricular dimensions associated with normal systolic and diastolic function, with or without increased left ventricular wall thickness).6 Left ventricular enlargement (>60 mm) occurs in ∼15% of highly trained athletes, and can be accompanied by an increase in absolute left ventricular wall thickness.6,7 These changes are dynamic in nature, develop rapidly after initiation of vigorous exercise, and are reversible with cessation of training. Although the changes in athlete's heart overlap significantly with age- and gender-matched sedentary controls, an important point to consider when examining a young athlete is the type of exercise, since endurance/aerobic training produces the most marked changes,8 and the pattern and extent of physiologically increased left ventricular mass varies with the nature of sports training. The most extreme increases in cavity dimension or wall thickness have been observed in athletes practicing rowing, skiing, cycling and swimming.9,10
Normal electrocardiographic changes in athletesMany preparticipation screening programs for sports include an ECG. Physicians responsible for the interpretation of these ECGs should be able to distinguish those changes that result from physiological adaptation to exercise, that are not pathological and reflect electrical and structural remodeling and autonomic nervous system adaptations to sustained physical activity. Up to 60% of athletes demonstrate ECG changes as a reflection of adaptation to exercise. Normal ECG changes are described in Table 1. The extent of these changes depends on the athlete's ethnicity, age, gender and level of training, as well as the type of training and even the type of sport. All these factors should be considered when looking at an athlete's ECG. Sports physicians and cardiologists should be aware of the normal changes on the ECGs of athletes, since a wrong diagnosis can have a significant impact on the life of a young athlete. At one extreme of the spectrum of ECG changes reflecting electrical remodeling, physicians may even indicate complete cessation of all sports, even if cardiac imaging (echocardiography or CMRI) shows no changes. The ESC guidelines for interpretation of the 12-lead ECG in the athlete divide changes on an athlete's ECG into two groups: Group 1 – common and training-related and Group 2 – uncommon and training-unrelated.2 Normal ECG changes may be summarized as follows:
- •
Sinus bradycardia, sinus arrhythmia, first-degree atrioventricular (AV) block and junctional escape rhythm
- •
Incomplete right bundle branch block (iRBBB)
- •
Early repolarization
- •
Isolated QRS voltage criteria for left ventricular hypertrophy
Normal ECG changes in athletes.
| 1. Sinus bradycardia (≥30 bpm) |
| 2. Sinus arrhythmia |
| 3. Ectopic atrial rhythm |
| 4. Junctional escape rhythm |
| 5. First-degree AV block (PR interval >200 ms) |
| 6. Mobitz type I (Wenckebach) AV block |
| 7. Incomplete right bundle branch block |
| 8. Isolated QRS voltage criteria for LVHa |
| 9. Early repolarization (ST elevation, J-point elevation, J waves, or terminal QRS slurring) |
| 10. Convex (‘domed’) ST segment elevation combined with T-wave inversion in leads V1–V4 in black/African/Caribbean athletes |
These normal, and exercise-related, ECG changes should be distinguished from much more uncommon, and pathological, changes such as ST-T repolarization abnormalities (T-wave inversion, ST-segment depression), pathological Q waves, left or right axis deviation, intraventricular conduction defects, ventricular pre-excitation, right ventricular hypertrophy and Brugada-like repolarization changes.
Sinus bradycardia and asymptomatic sinus pauses greater than 2 s (particularly during sleep) are common in athletes and are due to increased vagal tone. In the absence of symptoms such as fatigue, syncope or dizziness, a heart rate >30 bpm should be considered normal in a well-trained athlete.11 Additionally, up to 55% of well-trained athletes may have sinus arrhythmia, caused by an exaggerated response to inspiration and expiration.12 A junctional rhythm occurs when the depolarization rate of the AV node is faster than the sinus rate; however, sinus rhythm resumes with increasing heart rate. Increased vagal activity may also cause first-degree AV block (up to 35% of cases) and Mobitz-type I second-degree AV block (up to 10% of cases), and these changes resolve with faster heart rates during exercise.13
Incomplete right bundle branch block (iRBBB) (defined as a QRS duration <120 ms and a right bundle branch block pattern) is also a very common finding in athletes (35%–50%) compared with less than 10% in young healthy controls,2,14 and is reversible with deconditioning.14 This right ventricular conduction delay is possibly caused by enlarged right ventricular (RV) cavity size and RV remodeling, resulting in increased conduction time, and not by a delay within the specialized conduction system.15 The occurrence of iRBBB in an asymptomatic athlete with a negative family history and physical examination does not require further evaluation. Although typical iRBBB is uncommon in arrhythmogenic right ventricular cardiomyopathy (ARVC), particular attention should be paid to ST-T abnormalities in patients with iRBBB because ARVC may present with iRBBB with T-wave inversion in the mid-precordial leads beyond V2, low limb-lead voltages and premature ventricular beats with a left bundle branch block pattern.
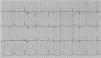
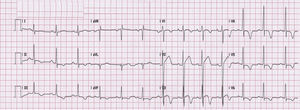
Early repolarization has long been recognized as a common, idiopathic and benign finding in young individuals. Its prevalence is estimated to be 1%–2% in the general population, with a clear male preponderance,16 and it is observed in 50%–80% of young athletes’ ECGs.17 Early repolarization is associated with the increased vagal tone observed in athletes and is a reversible phenomenon that disappears with deconditioning. The most common pattern seen in Caucasians is an elevated ST segment with an upward concavity, ending in a positive (‘peaked and tall’) T wave. These changes are more marked in V3–4, but maximal ST-segment displacement may also be seen in V5, V6, I and aVL or in the inferior leads (II, III and aVF). Early repolarization can be markedly different in athletes of African–Caribbean descent, in whom an elevated ST segment with an upward convexity is followed by a negative T wave in V2–V4 (Figure 1). If not interpreted in the appropriate context, an ECG with such changes in an African–Caribbean athlete may lead to misinterpretation of the ECG, with a consequent misdiagnosis and possibly withdrawal from sports. Normal repolarization changes in African–Caribbean athletes do not extend beyond V4. Thus, T-wave inversion in the lateral leads (V5–V6) is always considered an abnormal finding and requires additional testing to rule out hypertrophic cardiomyopathy (HCM) or other cardiomyopathies. These changes are modulated by the autonomic nervous system and vary with heart rate. Slowing of heart rate exaggerates ST-segment elevation whereas sinus tachycardia reduces and can even eliminate early repolarization changes. Late QRS slurring or notching with horizontal ST-segment elevation in the inferolateral leads has been associated with an increased risk of arrhythmic death in one study of middle-aged, non-athletic Finnish citizens.18 However, a significant percentage of young competitive athletes (25%–30%) show early repolarization with similar morphological features in either the inferior or lateral leads,19 a finding that is related to increased vagal tone. The currently available data are too scarce to assume a cause–effect relationship between this ECG pattern and the risk of malignant ventricular arrhythmias; however, in athletes presenting with syncope or cardiac arrest which remains unexplained after a detailed clinical work-up aimed to exclude cardiac causes and neuromediated mechanisms, the ECG pattern of early repolarization in the inferior and/or lateral leads, particularly when associated with prominent terminal QRS slurring, should raise the suspicion of underlying idiopathic ventricular fibrillation.2
As described previously, cardiac remodeling in athletes, among other changes, is associated with an increase in absolute left ventricular wall thickness, ventricular mass and increased chamber dimensions. These changes may be reflected on the 12-lead ECG as an isolated increase in QRS amplitude, with normal QRS axis, normal atrial and ventricular activation patterns and normal ST-segment and T-wave repolarization. Many factors can affect the QRS voltage in athletes, which is higher in males, black individuals and athletes engaged in endurance sports such as cycling, skiing and rowing/canoeing. Although anything between the left ventricular myocardium and the surface electrodes will affect QRS voltage, the presence of higher QRS voltage, without ST-T segment abnormalities, reflects the physiological LV hypertrophy associated with training and is benign. Other non-voltage criteria for LV hypertrophy, such as atrial enlargement, left axis deviation or a strain pattern of repolarization (ST-segment depression in V5–6, I and aVL), are not usually seen in athletes and should raise the suspicion of an underlying pathology, such as aortic valve disease, hypertension, or HCM. Although HCM can present with isolated increased QRS voltage this is a very uncommon finding.20
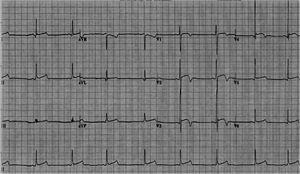
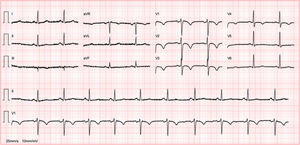
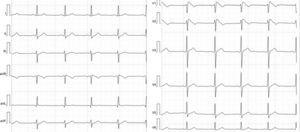
Abnormal and training-unrelated electrocardiogram changesThe most common causes of athlete deaths are HCM (up to 36% of cases) and coronary artery anomalies, together accounting for a little over 50% of SCD in competitive athletes.21 Other causes include dilated cardiomyopathy (DCM), ARVC (Figure 2) and cardiac ion-channel diseases such as long QT syndrome and Brugada syndrome (Figure 3). The ECG may disclose changes associated with these conditions (inverted T waves and ST-segment depression, pathological Q waves, Brugada-like repolarization changes and others) (Table 2). Such abnormalities are uncommon in athletes (<5%) and, when present, should always raise the suspicion of an underlying cardiac disease; further work-up is thus mandatory.2 ECG abnormalities of structural heart diseases that manifest with left ventricular hypertrophy, such as HCM, hypertensive heart disease or aortic stenosis, overlap only marginally with training-related ECG changes.2 An isolated QRS voltage criterion for LV hypertrophy is a very unusual pattern in pathological causes of LV hypertrophy such as HCM or hypertensive heart disease; in these patients, one or more additional non-voltage criteria such as atrial enlargement, left axis deviation, ST-segment and T-wave abnormalities, and pathological Q-waves are usually present, unlike in the physiological LV hypertrophy of athletes. HCM is a common genetic disease present in 1:500 of adults,22 although it appears to be less common in competitive athletes (1:1000–1:1500).23 HCM can be diagnosed with the ECG (Figure 4) in combination with echocardiography or CMRI. However, up to 6% of HCM patients have a normal ECG; this subset of patients appear to have a less severe phenotype with better cardiovascular outcomes.24 Based on these premises, the European guidelines for interpretation of 12-lead ECG in the athlete recommend that, regardless of personal and family history, athletes with non-voltage criteria for LV hypertrophy should undergo echocardiographic evaluation to exclude underlying structural heart disease.
Abnormal ECG changes in athletes and potential diagnoses.
| Abnormality | Criteria for further evaluation | Potential diagnosis |
|---|---|---|
| ST-segment depression | >0.5 mm below PR isoelectric line between J-junction and beginning of the T wave in V4–6, I and aVL OR >1 mm in any lead | HCM, DCM; CAD |
| RA enlargement and RV hypertrophy | P-wave amplitude 2.5 mm; Sokolow–Lyon voltage criteria for RV hypertrophy: R (V1)+S (V5) >10.5 mm OR R >7 mm in V1 OR R/S ratio >1 in V1 | HCM; congenital heart disease with RV pressure or volume overload |
| T-wave inversion | ≥1 mm in leads other than III, aVR and V1 | HCM; CAD; DCM; ARVD; pre-excitation syndrome; aortic valve disease; systemic hypertension; LV non-compaction |
| Q waves | >3 mm in depth and/or >40 ms duration in any lead except aVR, III, I and aVL | HCM; CAD |
| LBBB | QRS >120 ms and LBBB pattern | DCM; CAD |
| RBBB | QRS >120 ms and RBBB pattern | ARVD; Brugada syndrome; congenital heart disease |
| QTc interval | >470 ms in males or >480 ms in females; <340 ms in any athlete | Long or short QT syndrome |
| Brugada pattern | Early, high take-off (>2 mm), and downsloping ST-segment elevation (‘J-wave’) of either the coved (negative T-wave) or ‘saddle-back’ (positive T-wave) type in V1–2/V3. Consider provocation testing with flecainide to unmask the diagnostic type 1 Brugada pattern | Brugada syndrome |
ARVD: arrhythmogenic right ventricular dysplasia; CAD: coronary artery disease; DCM: dilated cardiomyopathy; HCM: hypertrophic cardiomyopathy; LBBB: left bundle branch block; RA: right atrial; RBBB: right bundle branch block; RV: right ventricular.
Other ECG changes, if present in a young athlete, should prompt further investigation to exclude cardiac disease. These include resting or exercise-induced ST-segment depression, right atrial enlargement and right ventricular hypertrophy, T-wave inversion, intraventricular conduction abnormalities (complete bundle branch block and hemiblock), abnormal QT interval (long or short) and Brugada-like ECG abnormalities. Special attention should be paid to T-wave inversion, which is not, as once believed, a common and training-related ECG change; the presence of T-wave inversion of >2 mm in two or more adjacent leads in an athlete is a non-specific warning sign of a potential cardiovascular disease at risk of SCD during sports.2 This finding may represent the initial phenotypic expression of an underlying cardiomyopathy, prior to the development of morphological changes detectable on cardiac imaging. T-wave inversion in the inferior (II, III and aVF) and/or lateral (I, aVL, V5 and V6) leads should raise the suspicion of ischemic heart disease, aortic valve disease, hypertensive cardiomyopathy, HCM or LV non-compaction,25 and post-pubertal persistence of T-wave inversion beyond V1 may reflect an underlying congenital heart defect with RV pressure and volume overload, ARVC26 or, uncommonly, an inherited ion-channel disease. Knowing that >2 mm T-wave inversion may identify athletes at risk for subsequent development of structural heart disease underscores the importance of continued clinical surveillance and follow-up with serial ECG and echocardiograms. Currently, the significance of minor T-wave changes such as flat and/or minimally inverted (<2 mm) T waves in two or more leads (mostly inferior and/or lateral) is unclear, and there is also no consensus regarding the presence of biphasic T waves. However, if the negative portion of the T wave is >1 mm in depth in two or more leads (excluding leads V1, aVR and V1), it is reasonable to consider this pattern as abnormal until more data are obtained.27 As described above, special attention should be paid to athletes of African/Caribbean descent in whom early repolarization is manifested as an elevated ST segment with upward convexity followed by a negative T wave (which can be profound) in V2 through V4. This finding is not considered abnormal and should be distinguished from other pathological patterns such as the presence of T-wave inversions in V1 through V4 with a flattened ST segment, as occurs in ARVC.28
Preparticipation screening of athletesAny screening program should be designed to take into account the frequency of the event, change or abnormality that it is desired to prevent or detect. Sudden cardiac death in athletes is an infrequent event, and only a very small proportion of those participating in sports are in fact at risk because of unsuspected cardiovascular disease. Screening programs should be driven by sound scientific principles based on evidence-based data and not by reaction to catastrophic events (such as the death of an athlete on the field).
The conditions most frequently responsible for sudden death in young athletes are HCM, a relatively common condition in the general population (1:50029), and coronary artery anomalies.21 Other, much rarer, conditions include ion channelopathies such as Brugada syndrome and long and short QT syndrome, ARVC, DCM and Marfan syndrome. These may amount to an estimated combined prevalence of 0.3% in general athlete populations. However, these diseases do not always cause changes on the ECG, which substantially reduces the sensitivity of the exam.
The greatest disadvantage of preparticipation screening of young athletes’ ECGs for abnormalities that may disclose a cardiac disease is the high number of false positives. Unawareness of the changes regarded as normal on young athletes’ ECGs can lead to these being classified as abnormal in up to 50% of athletes.2 Knowledge of the ECG changes associated with the type and intensity of exercise, race, age and gender can be expected to lower the traditionally high number of false positives, thus reducing unnecessary investigations.
In 1996, the American Heart Association (AHA) issued a scientific statement advocating universal cardiovascular preparticipation screening for high school and college athletes in an attempt to identify those at increased risk of cardiovascular events.30 The recommendations included a 12-point complete history and physical examination (including brachial artery blood pressure measurement) before competitive sports and reserved noninvasive testing such as a 12-lead ECG, echocardiogram, exercise testing, and cardiovascular consultation for athletes in whom any abnormality was detected. More recently, the AHA issued a scientific statement on the assessment of the 12-lead ECG as a screening test for detection of cardiovascular disease in healthy general populations of young people.31 The authors argue that, currently, there is insufficient information available to support the view that universal screening with 12-lead ECGs in asymptomatic young people (including competitive athletes) for cardiovascular disease is appropriate. The authors acknowledge that the ECG can promote detection of specific cardiovascular diseases and thereby benefit some individuals in a screening environment, but cannot be regarded as an ideal or effective test when applied to large healthy populations, and endorse the more widespread dissemination of automated external defibrillators.31 However, to recommend abandoning screening in favor of improving resuscitation is not the best formula for progress. Another argument against the feasibility of such a screening program is the absence of infrastructure for routine ECG screening that covers all the population of young athletes. Such an infrastructure should include experienced physicians specializing in sports medicine who are able to correctly interpret a large volume of young athletes’ ECGs, taking into consideration the normal and abnormal ECG changes in this population, and refer cases considered abnormal according to current ESC and AHA recommendations for further cardiovascular evaluation.2,3 The working group acknowledges, however, that screening with 12-lead ECGs, in association with comprehensive history-taking and physical examination, may be considered in relatively small cohorts of young healthy people 12–25 years of age, provided that the known and anticipated limitations of the 12-lead ECG as a population screening test are recognized.
However, the prescreening strategy of the ESC and the International Olympic Committee differs significantly from the American approach in that universal 12-lead rest ECGs are recommended for athletes <35 years. This recommendation relies heavily on the 25-year Italian experience of systematic ECG preparticipation screening of competitive athletes, a program that started in 1982. According to Corrado et al.,32 the annual incidence of SCD in athletes decreased from 3.6 deaths per 100000 person-years (1 death per year per 27777 athletes) in 1979–1981 to 0.4 deaths per 100000 person-years (1 death per year per 250000 athletes) in 2003–2004, an 89% reduction, with no change in mortality rates among the unscreened nonathletic population. Although this is the largest report to date on SCD in athletes, there are important limitations that should be taken into account.33 The study was a population-based observational report and not a temporally controlled comparison of screening versus non-screening in athletes; a separate analysis of the routine use of ECGs compared with more limited screening in identifying athletes at increased risk is not provided; the annual death rate before the initiation of the program was 1 per year per 27000 athletes, which is relatively high compared with other studies; the lowest annual death rate achieved with screening was 0.4 deaths per 100000 person-years (similar to the 0.44 sudden deaths per 100000 person-years reported for high school and college athletes in the USA from 1983 to 199334); and the event rates in the Italian study included all events, not those that occurred only with exertion.
Considering that the most common cause of SCD in athletes is HCM, this supports the strategy of ECG screening, since HCM can be reliably identified, or at least suspected, with an ECG in most HCM patients. In this particular case only a very small subset (∼6%) of patients with HCM, those with a normal ECG, would be missed. This group appears to have a better prognosis compared to HCM patients with ECG changes.24
The efficacy and efficiency of screening with ECG in athletes has not yet been fully demonstrated. As stated above, the efficiency of a screening program depends on the frequency of the condition or event for which it was designed to identify, and sudden death in an athlete, which has a high media profile, is a rare event. The ECG is not an ideal method for screening due to the high numbers of false positives and false negatives, and obviously the cost-effectiveness of this strategy depends on the specific health-care system in which it is implemented. The ECG is intended only as the first line in the screening process; it is not expected or claimed to be absolutely “diagnostic”. Moreover, conditions that can cause SCD, such as coronary artery anomalies or catecholaminergic polymorphic ventricular tachycardia, cannot be reliably identified by the currently recommended ECG screening, and identifying an athlete with a genetically based SCD condition may serve many others in the family. This is not just about preventing death on the athletic field.
To conclude, a large number of young athletes present with ECG changes, most resulting from adaptation to exercise, and only a very small number actually have changes that are considered abnormal and that prompt further investigation. Athletes with ECG changes due to cardiac adaptation to physical exertion should be reassured that they can continue to participate in competitive sports without additional investigation, in the absence of symptoms or a family history of cardiac disease or premature SCD.
Conflicts of interestThe authors have no conflicts of interest to declare.