Coronary optical coherence tomography has emerged as the most powerful in-vivo imaging modality to evaluate vessel structure in detail. It is a useful research tool that provides insights into the pathogenesis of coronary artery disease. This technology has an important clinical role that is still being developed. We review the evidence on the wide spectrum of potential clinical applications for coronary optical coherence tomography, which encompass the successive stages in coronary artery disease management: accurate lesion characterization and quantification of stenosis, guidance for the decision to perform percutaneous coronary intervention and subsequent planning, and evaluation of immediate and long-term results following intervention.
A tomografia de coerência ótica coronária surgiu como a modalidade de imagem in-vivo que permite a avaliação estrutural vascular mais detalhada. Trata-se de uma ferramenta valiosa em investigação, tendo contribuído para melhor entendimento da patogénese da doença coronária. Apresenta igualmente um papel importante na prática clínica, e o leque de sua aplicabilidade tem aumentado. Enquadrando na evidência disponível, discutimos neste artigo as principais aplicações da tomografia de coerência ótica coronária na prática clínica, que englobam as diferentes etapas na abordagem da doença coronária, incluindo a caracterização da lesão e quantificação da estenose, o papel na decisão de realizar angioplastia, o contributo na planificação da mesma e a avaliação dos resultados a curto e a longo prazo após a intervenção.
acute coronary syndrome
drug-eluting stent
fractional flow reserve
incomplete stent apposition
in-stent restenosis
intravascular ultrasound
minimal luminal area
minimal luminal diameter
optical coherence tomography
percutaneous coronary intervention
thin-cap fibroatheroma
Coronary angiography is the standard invasive imaging method for diagnosis of coronary artery disease and for guiding coronary interventional procedures.1 In addition to intravascular ultrasound (IVUS), optical coherence tomography (OCT) has emerged as an imaging modality able to evaluate the vessel structure in detail, for which angiography may not suffice.2–4 The OCT image is formed by the backscattering of emitted near-infrared light, creating cross-sectional images of the coronary vessel.2 Compared to IVUS, the wavelength used in OCT is shorter, enabling higher spatial resolution (10–20 μm axial resolution and 20–30 μm lateral resolution).2,5,6 However, except for calcium, penetration depth of OCT is lower than with IVUS, particularly for thrombotic and lipid components.2 Coronary OCT systems have evolved from first-generation time-domain systems to second-generation frequency-domain OCT.4 The latter produces images at higher frame rates with slightly deeper penetration, using a short, non-occlusive flush and rapid spiral pullback.2,4 We had the opportunity to perform the first OCT studies in Portugal. In addition to research purposes, we recognize the invaluable potential of OCT as a diagnostic technique and as an adjunctive tool for percutaneous coronary intervention (PCI).2,3
Data relevant to this topic have recently been published and we review the evidence on current clinical applications of OCT from a practical perspective. The potential use of OCT in the successive stages in coronary artery disease management is discussed, including morphologic lesion characterization and quantification of stenosis, guidance for the decision to perform percutaneous coronary intervention and subsequent planning, and evaluation of immediate and long-term results following PCI.
Morphologic lesion characterizationAnimal and human post-mortem studies have shown the ability of OCT to accurately characterize coronary atherosclerotic plaques.2,5 Due to its high spatial resolution, OCT has proved superior to other imaging modalities, including IVUS, for detecting different plaque components.2,5,6 A landmark post-mortem study showed a high sensitivity and specificity for detecting fibrous, fibrocalcific and lipid-rich plaques in histological specimens.5 OCT is currently the only method with sufficient resolution to accurately measure the fibrous cap.7 Historically, thin-cap fibroatheromas (TCFAs) are the substrate of approximately two-thirds of acute myocardial infarctions as presented in pathology series.8 Recently, this has been validated in vivo in the OCTAVIA study.9
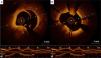
Furthermore, macrophage infiltration, which is a marker of plaque instability, may be identified using OCT.10 In acute coronary syndrome (ACS) OCT is useful for identification of plaque dissection, ulceration, and erosion, calcified nodules and thrombus7 (Figures 1 and 2). In addition, OCT can differentiate between red and white thrombi.11 A complete description of the appearance of atherosclerotic and thrombotic components on OCT is beyond the scope of this review and is reported elsewhere.12
OCT is particularly valuable in providing insights into the pathophysiological mechanisms of ACS and may help with the development of individualized therapeutic strategies.13 TCFA, plaque rupture and red thrombus have been detected in most patients with ST-elevation myocardial infarction and are more frequent in comparison to non-ST elevation ACS.14,15 However, not all ACS lesions showed plaque rupture and the presence of intact fibrous cap was associated with better prognosis.16,17 Moreover, plaque rupture, intracoronary thrombi, lipid-rich plaques and TCFAs were more frequent in culprit compared to nonculprit lesions.18
There are, however, some pitfalls in plaque characterization, mainly related to the low penetration depth. Penetration is lowest for thrombotic material, which may lead to signal-free shadowing, and non-protruding red thrombi may be misinterpreted as necrotic lipid pools due to a similar OCT signal pattern.12 Furthermore, in the majority of lesions an accurate measurement of lipid pool thickness cannot be performed.12
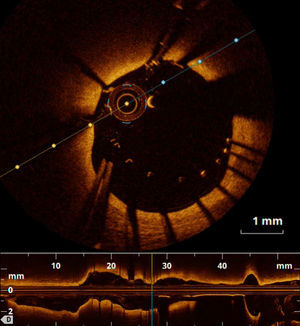
Stable coronary syndrome: predicting physiology and assessing stenosis severityOCT may be used to assess lesions of intermediate stenosis severity in vessels without a large size.4 The proposed thresholds of minimal luminal area (MLA) and minimal luminal diameter (MLD) for detecting a hemodynamically significant lesion are based on recent validations against fractional flow reserve (FFR), which is considered the gold standard for assessing hemodynamic significance.19–22 In most studies, FFR ≤0.80 was taken as the threshold and the derived cut-offs of MLA and MLD ranged from 1.59 mm2 to 2.54 mm2 and from 1.23 mm to 1.77 mm, respectively.20–22 Taking FFR <0.75 as the threshold, an MLA <1.91 mm2 and an MLD <1.35 mm have been identified as the best cut-off values.23 A recent consensus report suggests a MLA threshold of 1.95 mm2, which has moderate sensitivity and negative predictive value for hemodynamic significance.4,24 In small vessels lower thresholds should probably be used.24 In most studies, however, the correlation between FFR- and OCT-derived measurements was only moderate.20,21,23 Despite potentially higher precision in determining MLA, this simple cross-section value cannot predict physiology accurately, as shown in a recent meta-analysis.25 The ability of OCT to automatically segment the lumen through the entire pullback enables volumetric analysis of the vessel for the first time. Applying the physical principles of fluid dynamics, a better correlation with FFR was obtained by deriving virtual flow reserve.26
Importantly, MLA values obtained with OCT are consistently lower than with IVUS. It has been speculated that the absence of non-uniform rotation distortion on OCT allows for a more precise contour of the lumen.27
Percutaneous coronary intervention guided by optical coherence tomographyAdjunctive tool in decision-making for percutaneous coronary interventionFew data are available on the clinical impact of using OCT for guiding the decision to perform PCI, but the results are encouraging. In a single-center study, 90 patients with ambiguous or intermediate lesions underwent PCI if MLA was <3.5 mm2 or in the presence of thrombus.28 Post-dilatation or additional stent implantation was performed in cases of stent underexpansion, incomplete stent apposition (ISA), significant intraluminal tissue prolapse, or edge dissection extending beyond 200 μm.28 In addition to high procedural success, good clinical outcomes were reported at 4.6±3.2 months of follow-up, with 2.2% repeat revascularization and no stent thrombosis.28 An ongoing randomized trial (FORZA) is evaluating the feasibility of PCI guided by OCT in angiographically intermediate lesions with stenosis area ≥75% assessed by OCT, or 50–75% with MLA <2.5 mm2, or if a major plaque ulceration is detected.29
Recently, OCT has been used to guide treatment in ACS in a different way. The unique ability of the method to detect thrombus and plaque rupture adds an unprecedented level of confidence in determining the underlying mechanism of plaque instability.7 In a small study of 100 patients treated with thrombus aspiration followed by OCT, no stent was implanted if the occlusion was mostly thrombotic and no significant coronary narrowing was detected by OCT, provided that the patient was symptom-free and TIMI flow was ≥2 (20 patients).30 Follow-up OCT studies showed a “normal vessel” and there were no adverse events at 12-month follow-up. The safety and feasibility of medical management without stent placement in selected ACS patients with large thrombus burden detected by OCT has also been reported in other studies, in which OCT revealed lesion characteristics that were not disclosed by angiography and facilitated treatment decisions.31,32
Adjunctive tool for planning percutaneous coronary interventionSimilarly to IVUS, OCT may be used to help plan the intervention and, when used systematically, it has been reported to alter procedural strategy in over 80% of cases.33 OCT can provide highly reproducible measurements of lesion length and reference vessel lumen diameter, which may guide stent selection.4 Information on plaque components such as calcification may suggest the use of ancillary devices such as rotational atherectomy.3 OCT is the only method that can accurately image calcium thickness, and a combination of high thickness and circumferential distribution may identify non-dilatable lesions.3 Moreover, a lower calcium angle depicted by OCT correlates with asymmetric (eccentric) stent expansion and a higher calcium angle correlates with stent underexpansion.34 OCT may also be useful during complex procedures such as PCI of chronic total occlusions, where it may identify subintimal wire entrapment or double channels.4
Another potential use of OCT regarding PCI planning is risk stratification. Previous OCT studies have linked lesion morphology with periprocedural microvascular damage or myocardial infarction. In non-ST-elevation ACS, the presence of TCFA and the size of the lipid arc at the culprit plaque were predictors of no-reflow.35 The presence of TCFA was also associated with elevation of post-PCI myocardial necrosis markers, particularly if colocalized with spotty calcification.36 In addition, in-stent thrombus or dissection detected by OCT after PCI also predicted periprocedural myocardial infarction.37 Although these OCT findings could aid risk stratification before or during PCI, larger studies are needed to confirm the role of this clinical application.
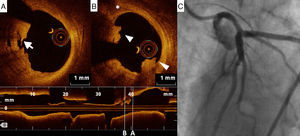
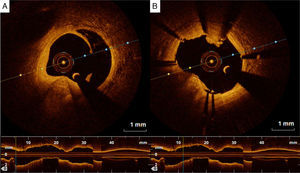
Adjunctive tool for post-stenting assessmentOne of the main contributions of OCT is in assessing stent expansion, sizing and apposition (defined elsewhere3,38) more accurately and with lower interobserver variability than angiography or IVUS. Stent underexpansion and undersizing have been linked to stent restenosis and stent thrombosis.39 The minimum stent area and lumen area of the reference vessel can be measured using OCT, enabling accurate estimation of stent expansion.3 ISA (Figure 3) delays neointimal coverage of the struts and is associated with stent thrombosis, although neointimal hyperplasia usually tends to reduce ISA over time.3,40 OCT can measure the distance between the struts and the vessel wall and quantify the number of struts with incomplete apposition.41,42 Stent apposition is thus assessed at the strut level.42
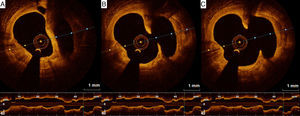
Regarding vascular injury after PCI, intimal dissection at the stent edges, small thrombi and tissue prolapse commonly occur following stent deployment (Figure 4).43 OCT is much more sensitive than IVUS for detecting these vascular responses.43 Nevertheless, their prognostic impact is controversial, as discussed below.
OCT may thus be useful after stent deployment, as it may prompt optimization with further stenting or high-pressure or larger-sized balloon inflation, with low procedural complication rates.44,45 Angiographic guidance for PCI in 335 patients was compared with angiographic plus OCT guidance in 335 patients in a propensity-score adjusted analysis (the CLI-OPCI study).45 OCT disclosed adverse features, including stent malapposition, stent underexpansion, lumen narrowing, thrombus, or edge dissection, requiring further stenting or additional balloon dilatation in 34.7% of patients. The OCT-guided approach resulted in lower adjusted risk of cardiac death or nonfatal myocardial infarction at 12 months of follow-up (OR 0.49, 95% confidence interval 0.25–0.96).45 In another study, even after achieving an optimal angiographic result following stenting, removal of in-stent thrombus detected by OCT using balloon dilatation was shown to be a safe approach.46 Of note, left main intervention can be performed with OCT guidance, and in a head-to-head comparison against IVUS, OCT identified more findings that prompted additional interventions.47 The DOCTORS trial is currently evaluating the utility of OCT for optimizing the results of coronary angioplasty in non-ST-segment elevation ACS, beyond angiography alone.48
Nevertheless, following stent deployment, minor stent malapposition with a short distance between the struts and the vessel wall, small thrombus, mild tissue prolapse, or minor stent edge dissection do not seem to be associated with worse prognosis.49 Improvement is expected during follow-up when these small non-flow limiting abnormalities are left untreated, without adverse impact on clinical outcome.49 Conversely, a dissection flap thickness >0.31 mm carries an adverse clinical impact in the long term.50 Regarding malapposition, some operators recommend no additional post-dilatation unless there is >200 μm distance between the stent and wall vessel at multiple strut locations.4
Follow-up evaluationStent coverageOCT has been used extensively to assess neointimal hyperplasia and strut coverage with different types of stents, placed using different stenting techniques, with or without adjunctive techniques. Different quantitative measurements may be obtained.3,51–55 Accuracy is greater than with IVUS; not only the completeness of individual strut coverage but also the thickness of coverage can be assessed.3,51 OCT studies have shown that a greater percentage of uncovered struts is associated with increased risk of major adverse events after drug-eluting stent (DES) implantation, a cut-off of ≥5.9% of uncovered struts on follow-up OCT having been reported.56
Stent restenosisQuantitative measurements such as percentage lumen obstruction can be obtained by OCT.3 In addition, different tissue patterns of in-stent restenosis (ISR) have been described, including layered, homogeneous and heterogeneous patterns.57 A heterogeneous pattern is more frequent in focal than in diffuse ISR and has been associated with the presence of fibrinoid or proteoglycans.57 In bare-metal stents, early ISR is usually homogeneous due to neointimal proliferation, which is rich in smooth muscle cells, while late ISR may have a heterogeneous appearance due to lipid pools, calcification and neovascularization, suggesting that neoatherosclerosis is the underlying mechanism.58 ISR of DES is typically characterized by a layered or heterogeneous intrastent tissue band and may be part of the spectrum of in-stent neoatherosclerotic changes, such as TCFA-containing neointima, particularly if the stent had been placed for at least 20 months.58
In a recent study on ISR, morphological assessment using OCT was useful for identifying lesions favorable for paclitaxel-coated balloon dilatation or DES placement.59 Of the 428 treated ISR lesions, a homogenous structure was associated with higher rates of repeated ISR and target lesion revascularization using plain balloon angioplasty alone compared with paclitaxel-coated balloon dilatation or DES placement (target lesion revascularization rates of 38.7%, 10.6% and 10.7%, respectively, in a follow-up of 211±40 days); conversely, no differences were found between the three treatment approaches in ISR lesions of heterogeneous appearance.59
Stent thrombosisThe mechanisms for stent thrombosis may be readily elucidated by OCT, as mentioned above. Strut malapposition or underexpansion, incomplete strut coverage, stent fracture, incomplete lesion coverage by the stent, edge dissection, in-stent neoatherosclerosis and ruptured neointima, particularly in areas of lipid-laden neointima, may be detected by OCT.4,60,61
Future directionsAs a clinical tool, OCT is currently potentially able to replace IVUS in every situation with the exception of diagnosis of lesions in the ostial left main or right coronary arteries, in which the flow in the aorta prevents adequate blood clearance.2–4 However, most of these patients should be assessed with FFR.2–4 Another exception is chronic total occlusions in which antegrade injections are of potential concern.2–4
The method continues to evolve, with pullback speeds now reaching 40 mm/s62 and the potential for a paradigm shift, with opportunities to save contrast compared to conventional angiography. The introduction of angiographic frame co-registration enables precise spatial location for stent implantation.63
OCT can replicate IVUS metrics that are essentially cross-sectionally derived, but most importantly allows for full volumetric analysis of the vessel.27 Virtual fractional flow reserve derived from OCT is a potentially disruptive technology in predicting physiology and assessing results after stenting.27
Finally, OCT is the most accurate method for follow-up assessment after placement of bioresorbable stents, considering their low opacity at fluoroscopy and the lower accuracy of IVUS.64
ConclusionThe current clinical applications of OCT encompass the successive stages in coronary artery disease management, from initial lesion evaluation to assessment of the final results of PCI, including accurate lesion characterization and quantification of stenosis, guidance for the decision to perform PCI and subsequent planning, and evaluation of immediate and long-term results following PCI.
Conflicts of interestThe authors have no conflicts of interest to declare.