Multiple left ventricular aneurysms (LVAs) are rare, especially in a young female. A 29-year-old woman presented vague symptoms. Multiple LVAs were revealed and confirmed on different imaging modalities, including chest radiography, echocardiography, contrast ventriculography and cardiac magnetic resonance imaging. Detailed work-up for probable etiologies including ischemic, infectious, inflammatory and autoimmune causes was negative. In the absence of angina, decompensated congestive heart failure, arrhythmias and embolism, the patient was managed conservatively, with excellent mid-term outcome.
Múltiplos aneurismas no ventrículo esquerdo (MAVE) são uma situação rara em especial numa jovem que apresentou queixas vagas. Vários MAVE foram revelados e confirmados em diferentes modalidades de imagem. A doente submeteu-se a radiografia de tórax, ecocardiografia, ecografia de contraste e ressonância magnética cardíaca. A investigação minuciosa de etiologias isquémica, infecciosa, inflamatória e autoimune foi negativa. Dada a ausência de angina, insuficiência cardíaca congestiva, arritmias e embolia, a doente foi tratada de forma conservadora apresentando um excelente prognóstico a médio prazo.
Left ventricular aneurysm (LVA) is usually defined as a segment of the ventricular wall that exhibits paradoxical systolic expansion; the term is generally reserved for a discrete, dyskinetic area of the left ventricular (LV) wall with a broad neck.1,2 It most frequently develops after myocardial infarction (MI).3 However, other causes may be infectious, inflammatory, metabolic or autoimmune in nature. Multiple idiopathic LVAs are distinctly unusual and rarely reported. The clinical spectrum ranges from asymptomatic to congestive heart failure (CHF) to life-threatening ventricular arrhythmias and cardiac arrest.3,4 Herein, we report the case of a young female with multiple large LVAs, managed conservatively in the absence of overt CHF, arrhythmias and embolism, with excellent outcome.
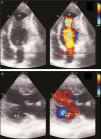
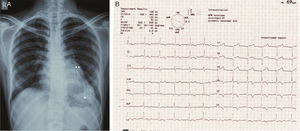
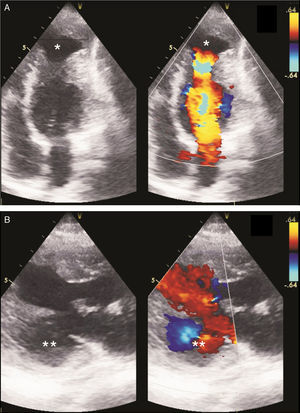
Case reportA 29-year-old woman presented with easy fatigability and chest discomfort for several months. These symptoms were insidious in onset and progressed little. There was no history of palpitations, syncope or dyspnea. She was hemodynamically stable with diffuse apex beat shifted downward and outward. The chest radiograph showed calcified LV apical and basal aneurysms. The electrocardiogram showed no pathological Q waves and normal precordial R-wave progression (Figure 1). Two-dimensional transthoracic echocardiography (iE33 xMATRIX, Philips Healthcare, Andover, MA, USA) showed moderate LV dysfunction; multiple LVAs (large apical aneurysm, moderate sized submitral aneurysm, and basal anterior aneurysm); normal contractility of the rest of the LV wall; and normal valves, other chambers and great vessels (Figure 2). The echocardiogram showed continuity of the myocardium of the aneurysms with the rest of the myocardium. Color flow Doppler showed flow in and out of the aneurysms.
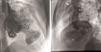
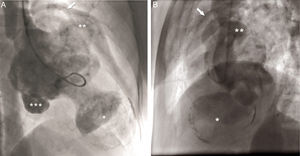
The coronary angiogram (Philips Medical Systems, Nederland B.V.) showed normal epicardial coronaries. Catheterization showed LV pressure of 114/3 mmHg. Systemic and pulmonary arterial, right atrial and ventricular pressures were also normal. The left ventriculogram showed a large LV apical aneurysm with a heavily calcified thrombosed distal part, a large aneurysm of the basal anterior wall with a smooth and calcified distal part, and a moderate sized submitral aneurysm. All the aneurysms had large necks, poor contractility and paradoxical systolic filling. Contrast clearance was slightly delayed by a beat (Figure 3).
Left ventriculogram. (A) Right anterior oblique view in diastole showing apical aneurysm (*), basal anterior aneurysm (**), submitral aneurysm (***) and normal left coronary artery branches (arrow); (B) lateral view in diastole showing LV apical aneurysm (*), basal anterior aneurysm (**) and normal left coronary artery branches (arrow).
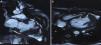
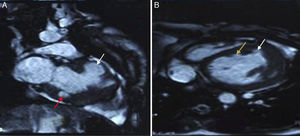
To further evaluate LV function and anatomy and the shape of the aneurysms and to differentiate between true and pseudoaneurysms, magnetic resonance imaging (MRI) (Achieva 1.5 T system, Philips Healthcare, Andover, USA) was performed. Using a dedicated cardiac coil, images were acquired with electrocardiographic gating and balanced turbo field echo/fast field echo in short-axis, vertical long-axis, 4-chamber and transverse planes, followed by perfusion studies and delayed contrast-enhanced imaging. These showed LV ejection fraction of 39.5% and stroke volume of 55.4 ml. Images were analyzed for the location of aneurysms, maximal internal width of the orifice, and maximal parallel internal diameter. The largest outpouching on the anterolateral wall of the LV showed thinning of the overlying myocardium (thickness 3.5 mm). The neck and maximum diameter were both 24 mm and the ratio of maximal internal width of the orifice and maximal parallel internal diameter was 1.0. There was no delayed pericardial enhancement. All these findings suggested that this was a true aneurysm. Other aneurysms on the anterior wall and submitral area were smaller and had similar characteristics (Figure 4). MRI ruled out inflammatory and infiltrative disorders.
The patient denied a history of rheumatic or systemic diseases, toxin exposure or chest trauma. There was no involvement of skin, mucosa or other organ systems. Family history was insignificant for CHF, cardiomyopathy, arrhythmias or sudden cardiac death. Serum levels of inflammatory markers (erythrocyte sedimentation rate and C-reactive protein), cardiac biomarkers and serum protein electrophoresis were normal. Investigations for Chagas disease, syphilis, sarcoidosis, tuberculosis, connective tissue diseases, and human immunodeficiency virus (HIV) were negative. Holter monitoring performed to screen for ventricular extrasystoles and tachyarrhythmias was normal. Abdominal ultrasound showed normal liver and kidneys.
Considering the idiopathic nature of the aneurysms and the absence of overt decompensated CHF, angina, arrhythmias and embolism, the patient was considered for medical management in the form of beta-blockers to prevent arrhythmias and warfarin to prevent systemic embolism. At 18 months of follow-up she was free of CHF, arrhythmias and embolism.
DiscussionLVAs are usually classified as congenital or acquired, i.e. arising from a cardiac or non-cardiac disorder.3 Acquired LVAs most frequently result from MI3,5 or coronary artery malformations such as fistulas.6 They may also be present in arrhythmogenic right ventricular cardiomyopathy,7 hypertrophic cardiomyopathy,8 and myocarditis.9 Underlying non-cardiac systemic diseases include sarcoidosis,10 Chagas disease,11 lupus erythematosus,12 Behcet's disease,13 tuberculosis,4 syphilis14 or HIV.15 As a rare complication, LVAs have also been observed in glycogen storage diseases,16 hyperimmunoglobulin-E syndrome17 and blunt chest trauma.18 Congenital LVAs (occurring in the perinatal period) have been detected as early as the second trimester of gestation19 and have been associated with severe comorbidities and intrauterine death. Idiopathic LVAs (without an identifiable underlying cause) are rare.3,20 Weakness of the LV wall, with herniation of the endocardium under the influence of ventricular pressure, and resulting in the formation of a fibrous walled aneurysm with calcium and thrombus deposits in its walls, sometimes with adhesions to the adjacent pericardium, has been suggested as the underlying pathogenesis.21 Idiopathic submitral LVA, of non-ischemic origin, a rare entity, is commonly described in young black Africans and sometimes in Caucasians.22 The higher prevalence in certain ethnic groups and the absence of a clear etiology suggest a congenital etiology.22 Idiopathic LVA appears to be caused by an abnormality in the junction between the cardiac muscle and the fibrous structure of the heart. It can vary in size from millimeters to several centimeters.22 These are anatomically distinct from congenital diverticula, which are defined as having only a narrow communication with the ventricle.15 Multiple idiopathic LVAs are distinctly unusual and are rarely reported.
As with aneurysms of atherosclerotic origin, these entities may be associated with a variety of symptoms, including chest discomfort,20 ventricular tachyarrhythmias leading to sudden cardiac death,3 angina,23 CHF,4 or recurrent thromboembolism.20 The tissue of the LVA has been described as inhomogeneous, with viable, normal myocytes, fibrotic tissue, and/or hypertrophic myocytes.3,4,24 This may well represent an arrhythmogenic substrate due to local conduction delay and dispersion of excitability and refractoriness, which may be augmented by catecholamines during exercise or mental stress.25 A study has demonstrated that inflammatory LV microaneurysms, often of viral origin, can be a cause of idiopathic ventricular tachyarrhythmias.26 In addition, large aneurysms interfere with LV performance through loss of contractile tissue due to mixture of scar tissue and viable myocardium and also by a paradoxical expansion,1 leading to CHF.
LVAs have been depicted and characterized by various imaging modalities.3 Radionuclide ventriculography and echocardiography can demonstrate LVAs more readily.1 A definitive diagnosis of LVA is best made non-invasively by echocardiography, which also helps in distinguishing a true from a pseudoaneurysm based on the demonstration of a narrow neck in relation to cavity size in the latter.27 The myocardium surrounding a true aneurysm is in continuity with the rest of the myocardium, as in our case, while there is no myocardium around a pseudoaneurysm, leading to a breach in the continuity of the normal myocardium.28 An abrupt discontinuity of the endocardial image between the aneurysm and adjacent normal myocardium is a characteristic finding with a pseudoaneurysm.14 True and pseudoaneurysms can both be non-contractile or dyskinetic and may contain thrombus.28,29 Color flow Doppler is useful in establishing the diagnosis as flow in and out of the aneurysm, as well as abnormal flow within it, can be detected. Pulsed Doppler imaging can reveal a to-and-fro pattern with characteristic respiratory variation in peak systolic velocity.1 However, echocardiography has the limitation of occasional failure to define the neck.30
Left ventriculography is another potential tool that can detect an abnormal bulge or dyskinetic wall motion in the LV contour during systole; however, it is an invasive method. True aneurysms have a wider neck, as in our case, in contrast to the narrow neck of a pseudoaneurysm.31,32 Contrast remains within the cavity of the pseudoaneurysm for several beats after injection.31,32 Coronary arteries can extend on the aneurysmal wall in a true aneurysm, while, in a pseudoaneurysm with disrupted myocardium, a cavity is created by blood and pericardium and the coronaries do not drape over this paraventricular chamber.32 A true aneurysm is less likely than a pseudoaneurysm to be secondary to MI, leading to abnormal patency of at least one of the coronary arteries. Other clues include a characteristic bulge and calcified LV silhouette on the chest radiograph, which is a specific finding but not sensitive.1 The presence of a discrete bulge anteriorly is more in favor of a true aneurysm.30 In the case of a pseudoaneurysm, the chest X-ray may show a pericardial mass with a characteristic notch at the border of the mass.14 In our case, by contrast, the borders were relatively smooth and a bulge was present anteriorly.
Recently, MRI has emerged as the preferred non-invasive technique for assessment of LV shape, the degree of aneurysm thinning, and resectability.33,34 MRI can distinguish between pericardium, thrombus, and myocardium, which are not easily distinguished by contrast ventriculography,30 and helps resolve uncertainty between a true and a pseudoaneurysm. Contrast-enhanced MRI provides additional information on myocardial tissue characteristics, perfusion, and viability.3 In the case of a true aneurysm, the tissue making up the wall of the aneurysm shows delayed myocardial enhancement. As the wall of a pseudoaneurysm is composed only of pericardium, it shows no delayed myocardial enhancement; however, its border does show enhancement.30 Pseudoaneurysms may show delayed pericardial enhancement.29 The ratio of maximal internal width of the orifice and maximal parallel internal diameter of 1.0 and the absence of delayed pericardial enhancement confirmed the aneurysms as true ones in the case presented.29 To elucidate the pathogenesis further, radionuclide imaging techniques can be used to assess myocardial viability (2-[fluorine-18]fluoro-2-deoxy-D-glucose positron emission tomography) and regional sympathetic innervation ([iodine-123]metaiodobenzylguanidine single photon emission computed tomography).3 Endomyocardial biopsy can demonstrate myocarditis, sarcoidosis, arrhythmogenic right ventricular cardiomyopathy, or storage disease.3 Programmed ventricular stimulation can help to induce clinically silent arrhythmias.3 As there was no history suggestive of hemodynamically significant arrhythmias, we assessed our patient with Holter monitoring only.
Treatment and prognosis of idiopathic LVAs are dependent on their size, location, and degree of valvular involvement, CHF functional class, and presence of ventricular tachyarrhythmias.3 Management strategies therefore range from antiarrhythmic drugs35 to ablation for ventricular tachycardia,36 implantation of a cardioverter-defibrillator,24 treatment of CHF3 or (less frequently) surgical aneurysmectomy.37 The latter is carried out to improve clinical manifestations, mostly CHF but sometimes also angina, embolization, and life-threatening tachyarrhythmias.1 However, prospective long-term follow-up studies comparing the different strategies are not available.3 Our patient was successfully managed with beta-blockers to prevent arrhythmias and oral anticoagulants to prevent thromboembolism. Idiopathic multiple LVAs in a young female demonstrated on ventriculography were reported to be treated conservatively with medical therapy with acceptable clinical course and without adverse clinical sequelae.20
ConclusionMultiple LVAs in a patient can be idiopathic in nature and have varied presentations. They can be managed conservatively in the absence of angina, decompensated CHF and tachyarrhythmias.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.