Speckle tracking echocardiography (STE) for two-dimensional (2D) strain analysis is a new tool to assess myocardial function. The aim of this study was to assess right atrial (RA) function using STE in patients with an atrial septal defect (ASD) before and one month after percutaneous closure.
MethodsWe prospectively examined 32 consecutive patients (nine male, 23 female) who underwent percutaneous transcatheter closure of a secundum ASD between June 2013 and December 2015. Echocardiography was performed on admission, prior to cardiac catheterization and then one month after ASD closure. Peak global RA longitudinal strain was analyzed by 2D-STE.
ResultsPatients’ mean age was 34.6±8.2 years. The mean diameter of the occlusive devices was 18.5±7.5 mm. Right ventricular (RV) end-diastolic diameters were significantly increased but decreased significantly after ASD closure (43±5 vs. 38±4 mm, p<0.05). Left atrial (LA) diameters (40±8 vs. 37±6 mm, p<0.05) decreased significantly after the intervention, whereas left ventricular (LV) end-diastolic diameters (45±5 vs. 46±4 mm, NS) remained unchanged. Tricuspid annular plane systolic excursion increased significantly (17.6±5.4 vs. 22.3±8.1 mm, p<0.05). After closure of the defect, a significant increase was observed in longitudinal RA strain (26.5±9.6% vs. 35.3±10.5%, p<0.001).
ConclusionsAfter percutaneous transcatheter closure of a secundum ASD, there was an increase in RA longitudinal strain. 2D-STE strain analysis appears to be helpful for the assessment of RA function and of response to correction of volume overload after percutaneous transcatheter closure of a secundum ASD.
O speckle tracking por ecocardiograma (STE) ou a avaliação do strain em 2 D são novas ferramentas para a avaliação da função miocárdica. O objetivo deste estudo foi avaliar a função da aurícula direita (AD) com o uso de STE em doentes com comunicação interauricular antes e um mês após o encerramento percutâneo.
MétodosAvaliamos prospetivamente 32 doentes consecutivos (nove homens, 23 mulheres) submetidos a encerramento percutâneo de comunicação interauricular (CIA) do tipo ostium secundum, entre junho de 2013 e dezembro de 2015. O ecocardiograma foi feito previamente ao procedimento e um mês após o encerramento da CIA. Foi avaliado o strain longitudinal global da AD (RALS) por 2D-STE.
ResultadosA idade média dos doentes foi de 34,6±8,2 anos. O diâmetro médio dos dispositivos empregados foi de 18,5±7,5 mm. Os diâmetros telediastólicos do ventrículo direito (VD) eram significativamente maiores e diminuíram após encerramento da CIA (43±5 versus 38±4 mm, p<0,05). O diâmetro da aurícula esquerda (AE) diminuiu significativamente após a intervenção (40±8 versus 37±6 mm, p<0,05), enquanto que o diâmetro telediastólico do ventrículo esquerdo permaneceu igual (45±5 versus 46±4 mm, NS). O TAPSE aumentou significativamente (17,6±5,4 versus 22,3±8,1 mm, p<0,05). Observamos ainda uma subida significativa do strain longitudinal da AD (26,5±9,6% versus 35,3±10,5%, p<0,001).
ConclusãoApós encerramento percutâneo de CIA ostium secundum, observou-se um aumento significativo do strain longitudinal global da AD.
Atrial septal defect (ASD) accounts for 25-30% of congenital heart defects diagnosed during adulthood.1 Ostium secundum ASD is the most common type, constituting 70% of all ASDs and 6-10% of all congenital heart defects. Left atrial (LA) and right atrial (RA) diameters and volumes are increased in ASD patients due to volume overload. ASDs cause a left-to-right shunt and chronic right chamber volume overload that can result in severe pulmonary hypertension and right heart failure. Secundum ASDs are centrally located and generally bounded by the superior and inferior limbic bands, which often makes these defects amenable to device closure.2,3
In the absence of fixed pulmonary hypertension, percutaneous ASD closure is associated with improvements in right ventricular dimensions, morphology and function and exercise physiology, and reverse right ventricular (RV) remodeling.3 However, such adaptations can be incomplete or delayed in adult patients, and atrial changes post-ASD closure are poorly understood.4
New echocardiographic methods have been developed to quantify left and right ventricular function and are important for diagnostic and prognostic assessment in various cardiovascular diseases.5,6 A new echocardiographic technique, two-dimensional (2D) speckle tracking echocardiography (STE), is capable of angle-independent tracking of myocardial deformation, enabling non-invasive and quantitative assessment of global and regional myocardial function.7
Myocardial STE measures tissue deformation within the myocardium expressed as a percentage change. Myocardial tissue lengthening and shortening give positive and negative strain values, respectively. Strain rate measures the local rate of myocardial deformation per time unit. The global strain or strain rate is calculated by computing deformation along the entire myocardial line length.8,9 This new echocardiographic method is frequently used to assess left ventricular (LV) myocardial function, but has rarely been used to examine RA and RV myocardial function. Myocardial strain and strain rate can be used to investigate RA myocardial function during each phase of the cardiac cycle. Padeletti et al. showed that RA speckle tracking analysis is significantly correlated with systolic pulmonary artery pressure and pulmonary arterial hypertension secondary to LV systolic dysfunction. They also found that assessment of RA longitudinal strain can identify pulmonary artery hypertension in congestive heart failure patients.10
The objective of the present study was to evaluate RA function by quantifying longitudinal RA strain in patients with chronic RV volume overload due to an ostium secundum ASD before and after percutaneous closure.
MethodsPatientsPatients with secundum ASD admitted to our center for percutaneous closure between June 2013 and December 2015 were included in this prospective controlled study. A total of 38 consecutive patients with secundum type ASD were investigated. The clinical indication for ASD closure was a hemodynamically significant left-to-right shunt (Qp/Qs >2.0) or echocardiographic signs of right heart dilation or shunt-related symptoms. Patients with a stretched ASD larger than 36 mm, those with inadequate atrial septal rims to permit stable device deployment, those in whom the defect was close to the AV valves, coronary sinus or vena cavae, and those with sinus venosus or primum ASD, pulmonary vascular resistance greater than eight Wood units despite 100% oxygen, other concomitant congenital heart disease, valvular heart disease, coronary artery disease, LV systolic dysfunction, atrial fibrillation, or hypertension were excluded. Six patients were not suitable for percutaneous ASD closure and so the study population consisted of 32 patients (23 female, nine male, mean age 41±13 years) with secundum type ASD and in sinus rhythm who underwent successful percutaneous ASD closure.
The study participants underwent clinical and echocardiographic assessment before and one month after percutaneous closure ASD. The study was approved by the local ethics committee of our hospital.
Echocardiographic examinationAll patients underwent comprehensive transthoracic echocardiographic examinations at rest according to the American Society of Echocardiography guidelines,11 using a Philips EPIQ 7C ultrasound system (Philips Healthcare, Andover, MA, USA) equipped with a multifrequency transducer (3-8 MHz) and tissue harmonic imaging capability. A single-lead electrocardiogram was recorded continuously and 2D echocardiographic images of the right atrium and right ventricle were obtained in apical 4-chamber view at end-expiration. Care was taken to capture the entire right atrium, allowing for more reliable delineation of the atrial endocardial border. The frame rate was set at between 40 and 80 frames per second. At least three consecutive cardiac cycles of 2D echocardiographic images recorded in apical 4-chamber view were stored in order to select the images with the best quality for off-line speckle tracking analysis.12
Two-dimensional speckle tracking analysisTwo-dimensional strain and strain rate analysis is a reliable technique for tracking of myocardial movement and deformation. Standard grayscale images are analyzed with a dedicated software package that focuses on specific speckle patterns and follows them on a frame-by-frame basis during a cardiac cycle.5 In contrast to Doppler-derived indices, STE has the advantage of being angle-independent, and is less affected by reverberations, side lobes and dropout artifacts.12 STE is frequently used to assess LV function and in recent years has been used to quantify longitudinal myocardial LA and RA deformation dynamics.13,14
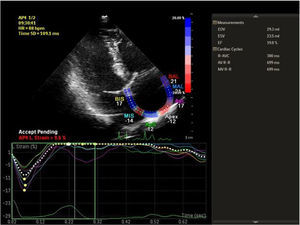
Speckle tracking analysis was performed by a single experienced and independent investigator (OO) who was blinded to the clinical data, using commercially available semiautomated 2D wall motion tracking software (Philips Healthcare Systems). To assess RA myocardial function, the RA endocardium was manually traced when the right atrium was at its maximum volume, just before tricuspid valve opening, as identified in apical 4-chamber view. The RA basal septal, basal lateral and apical borders were manually traced, followed by automatic tracing of the endocardial and epicardial borders, thus delineating a region of interest composed of six segments. After analysis of segmental tracking quality and manual adjustment of the region of interest, longitudinal strain curves were generated by the software for each atrial segment. A cine loop preview feature enabled visual confirmation that the internal line followed the movements of the RA endocardium throughout the cardiac cycle. If tracking of the RA endocardium was unsatisfactory, manual adjustments of the region of interest size were performed to ensure optimal tracking (Figure 1). RA longitudinal strain was measured in six segments of the atrium. As previously described, peak RA longitudinal strain at the end of the RA reservoir phase was calculated by averaging the values for all RA segments to yield global RA longitudinal strain.10,12
Percutaneous atrial septal defect closure procedurePercutaneous ASD closure was performed under general anesthesia utilizing fluoroscopic and multiplane transesophageal echocardiographic (TEE) guidance. Closure was achieved using Figulla Flex II ASD devices (Occlutech GmbH, Jena, Germany). The larger diameters of the ASD were measured at different angles of 2D and three-dimensional TEE and device size was determined by adding 2-4 mm to the larger ASD diameter. In addition, the distance to the defect was required to be at least 5 mm from the mitral valve, right upper pulmonary vein, coronary sinus, tricuspid valve, inferior vena cava, and superior vena cava. In the case of deficient aortic rim (<5 mm), percutaneous closure was performed if the other rims had sufficient length. Balloon measurements were not performed routinely.12,13 Post-interventional treatment included 100 mg/day aspirin and 75 mg/day clopidogrel for six months.
Statistical analysisStatistical analysis was performed with the SPSS statistical package, version 12.0 (SPSS Inc.). Continuous variables were expressed as mean ± SD and categorical variables were expressed as percentages. Intra-observer variability was calculated as the absolute difference between two measurements in percentage of their mean. The Student's t test and the chi-square test were used for comparison of data, as appropriate. Differences between baseline and follow-up were analyzed by the paired sample t test. Pearson correlation analysis was used to determine the relationship between RA longitudinal strain and other echocardiographic parameters. A p value <0.05 was considered statistically significant.
ResultsBaseline demographic characteristics and procedure-related clinical parameters of the study population are summarized in Table 1. The mean native secundum ASD diameter on color Doppler echocardiographic assessment was 14±5 mm (10-22 mm) and the mean diameter of the closure devices used was 18.5±7.5 mm (14-28 mm). Percutaneous closure was performed successfully in all 32 patients. There was no residual ASD in any of the patients on echocardiographic assessment at one month after the procedure. The echocardiographic measurements are given in Table 2. RV end-diastolic diameters were significantly increased but decreased significantly after ASD closure (43±5 vs. 38±4 mm, p<0.05). LA diameters (40±8 vs. 37±6 mm, p<0.05) decreased significantly after the intervention, whereas LV end-diastolic diameters (45±5 vs. 46±4 mm, NS) remained unchanged. Tricuspid annular plane systolic excursion (TAPSE) increased significantly (17.6±5.4 vs. 22.3±8.1 mm, p<0.05). After closure of the defect, a significant increase was observed in longitudinal RA strain (Table 2). Intra-observer variability for RA longitudinal strain was 3.2+1.6%.
Clinical characteristics and echocardiographic measurements in patients with atrial septal defect.
| Variable | |
|---|---|
| Age, years | 34.6±8.2 |
| Gender, female/male | 23/9 |
| SBP, mmHg | 123±15 |
| DBP, mmHg | 69±9 |
| HR, bpm | 93±11 |
| ASD diameter, mm | 14±5 |
| Device diameter, mm | 18.5±7.5 |
| Qp/Qs ratio | 2.6±0.7 |
| BMI, kg/m2 | 1.89±0.16 |
ASD: atrial septal defect; BMI: body mass index; bpm: beats per min; DBP: diastolic blood pressure; HR: heart rate; SBP: systolic blood pressure.
Effects of atrial septal defect closure on echocardiographic parameters.
| Variable | Pre-procedure | One month post-procedure | p |
|---|---|---|---|
| LVEDD, mm | 45±5 | 47±4 | NS |
| LVESD, mm | 28±5 | 29±4 | NS |
| LVEF, % | 58±3 | 63±4 | <0.05 |
| LA diameters, mm | |||
| Antero-posterior | 37±8 | 34±5 | <0.05 |
| Medio-lateral | 40±8 | 37±6 | <0.05 |
| Apico-basal | 48±5 | 47±6 | <0.05 |
| RA diameters, mm | |||
| Medio-lateral | 43±7 | 37±6 | <0.05 |
| Apico-basal | 50±6 | 46±5 | <0.05 |
| RVEDD, mm | 43±5 | 38±4 | <0.05 |
| PASP, mmHg | 51.4±16.3 | 37.3±12.7 | <0.05 |
| TAPSE, mm | 17.6±5.4 | 22.3±8.1 | <0.05 |
| RA longitudinal strain, % | 26.5±9.6 | 35.3±10.5 | <0.001 |
LA: left atrial; LV: left ventricular; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; PASP: pulmonary artery systolic pressure; RA: right atrial; RV: right ventricular; RVEDD: right ventricular end-diastolic diameter; TAPSE: tricuspid annular plane systolic excursion.
Correlation analysis was performed between post-ASD and pre-ASD parameters. The delta value was calculated as post-ASD parameter minus pre-ASD parameter. The analysis showed negative correlations between delta RA longitudinal strain and delta RV diameter, delta RA diameter and delta pulmonary artery systolic pressure (PASP). Also, a positive correlation was found between delta RA longitudinal strain and delta TAPSE. However, there was no correlation between delta RA longitudinal strain and delta LVEF, delta LV diameter or delta LA diameter (Table 3).
Correlations between right atrial longitudinal strain and other echocardiographic parameters in patients with atrial septal defect.
| Parameter | Delta (post-ASD minus pre-ASD) | Pearson's correlation coefficient (r) | p |
|---|---|---|---|
| RVEDD | -4.9±4.23 | -0.573 | 0.022 |
| RA diameter | -5.23±5.76 | -0.154 | 0.041 |
| LVEDD | +2.19±4.45 | 0.126 | 0.061 |
| LA diameter | -2.25±4.9 | -0.132 | 0.068 |
| LVEF | +5.13±3.38 | 0.119 | 0.047 |
| PASP | -14.1±13.8 | -0.497 | 0.039 |
| TAPSE | +4.7±6.9 | 0.514 | 0.027 |
ASD: atrial septal defect; LA: left atrial; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; PASP: pulmonary artery systolic pressure; RA: right atrial; RVEDD: right ventricular end-diastolic diameter; TAPSE: tricuspid annular plane systolic excursion.
The right atrium has multiple important roles during the cardiac cycle: it functions as a reservoir for systemic venous return when the tricuspid valve is closed, provides a passive conduit for blood flow during early diastole when the tricuspid valve opens, and actively expels blood during late diastole with atrial contraction.7 The right atrium has an unusual geometry and thin walls. Assessment of atrial dimensions and volume using 2D echocardiographic parameters has limitations. Measurement of atrial dimensions with M-mode and 2D echocardiography assumes the atria have an elliptical geometry,15 but this is clearly not completely true, and the variable and non-uniform geometry of the atria leads to significant differences in the measurement of atrial volumes using the biplane area-length method and biplane modified Simpson's rule.15 Anatomical and functional assessment of the RA is therefore difficult.16–18 In previous studies, 2D transthoracic echocardiographic measurements in patients with ASD demonstrated enlarged right atrium, right ventricle, and pulmonary arteries. These usually correlate with the magnitude of the left-to-right shunt.19 In the past decade, the novel echocardiographic technique of strain imaging has been used to make more accurate assessments of myocardial function.20 STE has been used in a wide range of cardiovascular conditions, including hypertension, heart failure and myocardial infarction.21,22 Conventional ways of determining systolic function rely on visual assessment of wall motion and changes in volume. However, STE measures actual tissue deformation within the myocardium.11
One of the new applications of STE is in assessment of RA myocardial function. The RA reservoir phase is a dynamic phase of the cardiac cycle. RA deformation is dependent on preload and afterload, which affect the right ventricle and consequently influence the right atrium. We therefore assessed RA myocardial function in patients with percutaneous ASD closure by focusing on the reservoir phase.
Two-dimensional STE assessment of RA myocardial function is feasible throughout the cardiac cycle. Peak RA longitudinal strain determines the reservoir phase.7 Padeletti et al. reported that RA STE significantly correlated with pulmonary pressure. They also found that RA assessment with STE can predict pulmonary artery hypertension in heart failure patients.10 We demonstrated that peak RA longitudinal strain increased significantly after percutaneous closure of secundum ASD. Our study is the first to assess the effect of percutaneous ASD closure on RA function with STE. Sakata et al. reported that RA longitudinal strain was useful for noninvasive assessment of RA dysfunction in patients with pulmonary artery hypertension. They also found that peak global RA longitudinal strain was correlated with cardiac index. Peak global RA longitudinal strain can be thus useful for the assessment of RA overload and dysfunction in pulmonary artery hypertension. This study showed an inverse correlation between PASP and peak global RA longitudinal strain.7
In our study, there was improvement in multiple echocardiographic parameters, including RV end-diastolic diameter, RA diameter, RV/LV end-diastolic diameter ratio, LA diameter and PASP at one month post-procedure. LV end-diastolic diameter and LV end-systolic diameter remained unchanged in the first month post-procedure, while LVEF and TAPSE were significantly increased. In this study, there was a positive correlation between delta RA longitudinal strain and delta LVEF and TAPSE and a negative correlation between delta RA longitudinal strain and delta RV myocardial performance index, RV diameter, RA diameter and PASP. Our finding of decreased RA and LA dimensions after percutaneous ASD closure is in agreement with similar reports in previous studies. For example, Akula et al. found a significant decrease in RV volumes in the first month after ASD closure that continued up to six months,23 and Veldtman et al. found that right heart morphology undergoes rapid improvement within one month of percutaneous ASD closure.24 Atashband et al. showed that percutaneous ASD closure in adults is effective, leading to reverse remodeling and better functional capacity,25 while Thilén et al. found that cardiac remodeling after ASD closure in the adult is common and is generally completed within the first half year after closure.26
Study limitationsSTE can be used for myocardial assessment in all dimensions (longitudinal, radial, and circumferential). In our study, we measured RA longitudinal strain only. Only one cardiac cycle was studied, and so respiratory variations may be a source of sample error. The software used for STE analysis was originally designed for the left ventricle, but in the present study it was applied to the right atrium. Defects in the atrial septum and the presence of an occlusion device may have affected RA longitudinal strain values.
ConclusionVolume overload is associated with decreased strain values, which return to normal when the volume overload is abolished. In this study, RA longitudinal strain measured by 2D-STE was useful to assess the effect of percutaneous ASD closure on RA dysfunction in the reservoir phase. STE can be considered a feasible and reproducible noninvasive technique for the assessment of RA longitudinal deformation dynamics in patients with ASD.
Conflicts of interestThe authors have no conflicts of interest to declare.