This article reviews the major pharmacologic features of, and clinical evidence on, adjuvant medical therapy in patients with ST-elevation myocardial infarction (STEMI) treated with primary percutaneous coronary intervention. These drugs include oral antiplatelets (aspirin and P2Y12 inhibitors such as clopidogrel, prasugrel and ticagrelor), intravenous antiplatelet agents (the P2Y12 inhibitor cangrelor, GP IIb/IIIa inhibitors such as abciximab, eptifibatide and tirofiban), and intravenous anticoagulant agents (unfractionated heparin, low molecular weight heparin and bivalirudin).
Neste artigo foram analisadas as principais características farmacológicas e a evidência clínica do uso de terapêutica médica adjuvante em doentes com enfarte do miocárdio com supradesnivelamento do segmento ST tratados com angioplastia primária. Esses fármacos incluem antiagregantes orais (ácido acetilsalicílico e inibidores de P2Y12, tais como clopidogrel, prasugrel, ticagrelor), antiagregantes endovenosos (o inibidor P2Y12 cangrelor, inibidores GpIIbIIIa, como o abciximab, eptifibatide e tirofiban) e agentes anticoagulantes de uso endovenoso (heparina não fracionada, heparina de baixo peso molecular e bivalirudina).
The acute standard of care for ST-elevation myocardial infarction (STEMI)1–3 includes activation of a STEMI care network, administration of adjuvant medical therapy, and reperfusion by primary percutaneous coronary intervention (PCI).
Developments in organizational aspects of health care, such as the Stent for Life initiative, and in reperfusion therapy, have established primary PCI as the current first option for the treatment of patients with STEMI, due to its impact on prognosis.4,5
The improvements now seen in STEMI care are closely related to advances in adjuvant medical therapy in recent decades, particularly antithrombotic therapy.
In this article, we present a narrative review describing the main pharmacologic characteristics of, and clinical evidence on, adjuvant medical therapy used in the acute phase of STEMI.
ReviewAspirin (Acetylsalicylic Acid)Aspirin acts as a platelet inhibitor at lower doses (75-325 mg), but has antipyretic and anti-inflammatory effects at higher doses.
The landmark trial for antiplatelet treatment in patients with acute myocardial infarction (MI) was the 1988 Second International Study of Infarct Survival (ISIS-2).6
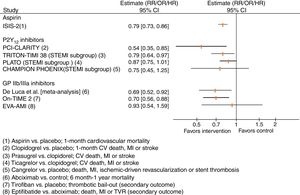
In this trial, the administration of 162.5 mg aspirin for 30 days achieved a 21% relative risk reduction (RRR) in vascular mortality compared with placebo (Figure 1), which was similar to the effect of streptokinase alone (RRR 23%). A reduction of 39% in vascular mortality was observed when aspirin was added to streptokinase.6 Overall, aspirin treatment showed an absolute risk reduction in vascular mortality of 2.4% and a number needed to treat of 42 patients within five weeks to prevent one vascular death, compared with placebo.
Results of selected trials assessing antiplatelet agents in patients with ST-segment elevation myocardial infarction. CI: confidence interval; CV: cardiovascular; GP: glycoprotein; HR: hazard ratio; MI: myocardial infarction; OR: odds ratio; RR: relative risk; STEMI: ST-segment elevation myocardial infarction; TVR: target vessel revascularization.
During the acute presentation of STEMI, the guidelines recommend a loading dose of aspirin of 150-300 mg orally or 80-150 mg intravenously with lysine acetylsalicylate in order to ensure inhibition of serum thromboxane B2 synthesis.1,3,7
The issue of aspirin dosing was further analyzed in the Clopidogrel and Aspirin Optimal Dose Usage to Reduce Recurrent Events−Seventh Organization to Assess Strategies in Ischemic Syndromes 7 (CURRENT-OASIS 7) trial, which included 25 086 patients with acute coronary syndromes (29% of whom, about 7000 patients, presented with STEMI).8 Patients randomized to high-dose aspirin (300-325 mg daily) for seven days showed similar rates to the low-dose group (75-100 mg daily) for the composite outcome of 30-day cardiovascular mortality, non-fatal MI or non-fatal stroke. The secondary outcomes were also similar between the interventions, with the exception of a lower incidence of recurrent ischemia in the high-dose aspirin arm. Minor bleeding events were increased with high-dose aspirin but the risk of major bleeding was similar with the different doses.8
P2Y12 inhibitors: clopidogrel, prasugrel, ticagrelor and cangrelorClopidogrelThe benefits of the addition of a second antiplatelet agent in addition to aspirin in STEMI patients are clearly demonstrated by clopidogrel. Clopidogrel is a second-generation member of the thienopyridine drug class, which inhibit binding of ADP to P2Y12 receptors in platelets and thus prevent ADP-related glycoprotein (GP) IIb/IIIa receptor activation.
Clopidogrel is a prodrug and requires a two-step activation process in the liver in which it is converted by cytochrome P450 (CYP) enzymes into an intermediate metabolite and a final active metabolite. CYP2C19 is one of the most important CYPs involved in clopidogrel metabolism. The concomitant use of CYP2C19 inhibitors (omeprazole, fluoxetine, or azole antifungal agents) leads to decreased levels of the active metabolite, but the clinical relevance of this decrease has not been clearly determined.9 Thus, alternative drugs should be considered before prescribing CYP2C19 inhibitors.
Clopidogrel as Adjunctive Reperfusion Therapy - Thrombolysis In Myocardial Infarction (CLARITY-TIMI 28) was a placebo-controlled trial designed to determine whether clopidogrel added to standard antiplatelet therapy with aspirin (loading dose plus maintenance dose of 75-162 mg/day) would further improve the prognosis of adult patients (18-75 years old) managed with fibrinolysis.10 A total of 3491 patients presenting within 12 hours of MI intended for treatment with a fibrinolytic agent were enrolled and randomized to receive either aspirin alone or aspirin plus clopidogrel (300 mg loading dose followed by 75 mg/day). The addition of clopidogrel to aspirin showed an RRR of 36% for occluded infarct-related artery, death or MI by the time of angiography, and a 20% RRR for cardiovascular mortality, MI or urgent revascularization due to recurrent ischemia at 30 days.10
The PCI-CLARITY study was a subgroup analysis of CLARITY-TIMI 28, with 1863 patients undergoing PCI a median of three days after randomization. All patients in this subgroup received a loading dose of 300 mg, then 75 mg once daily of open-label clopidogrel before PCI. Pretreatment with clopidogrel after fibrinolytic therapy and before PCI resulted in a 46% RRR of the composite of cardiovascular death, recurrent MI or stroke compared to placebo (Figure 1).11
The clopidogrel arm of ClOpidogrel and Metoprolol in Myocardial Infarction Trial/Second Chinese Cardiac Study (COMMIT-CCS2)12 enrolled 45 852 patients with MI (93% with STEMI or MI with left bundle branch block) randomized to receive either clopidogrel or placebo in addition to aspirin 162 mg/daily until discharge or up to four weeks in hospital. Clopidogrel significantly reduced all-cause mortality and the composite outcome of death, reinfarction or stroke, with RRRs of 7% and 9%, respectively, after a mean treatment period of two weeks.
The rates of major bleeding found in these two trials (CLARITY-TIMI 28 and COMMIT-CCS2) were not significantly increased.10,12
Clopidogrel loading doses (600 mg vs. 300 mg) were compared in 201 STEMI patients undergoing primary PCI in the Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Myocardial Infarction (ARMYDA-6 MI) trial.13 The primary outcome, the area under the curve of cardiac biomarkers as a surrogate of myocardial infarct size, was significantly lower with the 600 mg loading dose than with the 300 mg loading dose. There were also fewer 30-day major adverse cardiovascular events but bleeding complications were similar between the different loading doses.13
The CURRENT-OASIS 7 trial (see section on aspirin, above), with a 2-by-2 factorial design, also assessed the clinical impact of high-dose clopidogrel (loading dose 600 mg, six days at 150 mg/day, followed by 75 mg/day) vs. standard dose (loading dose 300 mg, followed by 75 mg/day).8 Overall, high-dose clopidogrel did not reduce 30-day cardiovascular mortality, MI or stroke, but in the subgroup of patients who underwent PCI (STEMI and non-STEMI) the risk of these major adverse cardiovascular events was significantly decreased, mainly due to the reduction of MI. High-dose clopidogrel increased the risk of major bleeding.8
PrasugrelPrasugrel, another member of the thienopyridine drug class, is an irreversible P2Y12 inhibitor that overcomes some of clopidogrel's limitations, particularly the degree of its antiplatelet effect and time to onset.
Prasugrel is rapidly hydrolyzed to a thiolactone in the intestine, and then after absorption is converted by CYP3A4 and CYP2B6 and to a lesser extent by CYP2C9 and CYP2C19 to the active metabolite, which reaches peak plasma concentration 30 min after administration.
The benefits of prasugrel (loading dose 60 mg, followed by 10 mg once daily) instead of clopidogrel in addition to aspirin (at any dose from 75 to 162 mg) were shown by the TRITON-TIMI 38 trial, which enrolled 13 608 patients with acute coronary syndromes managed invasively with PCI.14
Prasugrel reduced the relative risk of the composite outcome of cardiovascular death, non-fatal MI and non-fatal stroke by 19% compared with clopidogrel. The results were mainly driven by reduced risk for non-fatal MI (RRR 24%), and the overall clinical impact of prasugrel was not different within the subgroup of 3534 STEMI patients (p=NS for interaction); the RRR in this subgroup was 21%. There was no mortality benefit with prasugrel and the bleeding risk was significantly higher according to the study definition of major bleeding (TIMI major bleeding not related to coronary artery bypass grafting [CABG]).14 The risk of death, non-fatal MI, non-fatal stroke and non-CABG-related TIMI major bleeding was significantly higher in patients with a history of stroke or transient ischemic attack, and thus prasugrel is not indicated for these patients. Other subgroups for which specific concerns were raised were the elderly (≥75 years old) and low-weight individuals (<60 kg), particularly regarding their bleeding risk with 10 mg prasugrel daily. Current regulatory documents recommend a careful individual benefit/risk assessment and if prescribed the maintenance dose should be 5 mg daily for such patients.
TicagrelorTicagrelor is an oral cyclopentyltriazolopyrimidine that reversibly and non-competitively inhibits ADP-induced signaling downstream of P2Y12 receptors. Compared to clopidogrel, ticagrelor showed a favorable profile regarding time to onset and degree of antiplatelet effect.
Ticagrelor reaches peak plasma levels between 90 min and three hours and peak platelet inhibition in two hours.
The pivotal trial of ticagrelor in ACS was PLATO (a Study of PLATelet Inhibition and Patient Outcomes), in which dual antiplatelet treatment with aspirin and ticagrelor was compared with aspirin and clopidogrel. The trial included a total of 18 624 patients with ACS. Both P2Y12 inhibitors were given in a loading dose (ticagrelor 180 mg; clopidogrel 300-600 mg) and a maintenance dose (ticagrelor 90 mg twice daily; clopidogrel 75 mg once daily). Ticagrelor showed a significant 16% RRR in cardiovascular mortality, MI and stroke, due to decreases in cardiovascular mortality and MI. Overall major bleeding risk was not significantly increased, but non-CABG-related major bleeding was significantly increased.
This trial included 7544 patients with STEMI, and the subgroup's result was similar to the non-STEMI population (p=0.29 for interaction). It is noteworthy that ticagrelor had less effect in North America than in the rest of the world.15 Other than chance alone or a true regional difference, one possible explanation is the fact that the aspirin maintenance dose was on average higher in North America. A significant statistical interaction was observed favoring the interaction of low-dose aspirin with ticagrelor. The recommended dose of aspirin to be taken with ticagrelor is 75-150 mg, preferably lower doses.
The main adverse events associated with ticagrelor are dyspnea and bradyarrhythmias. The incidence of dyspnea in the ticagrelor arm in the PLATO trial was 13.8%. It was usually non-severe, transient and not related to cardiac, pulmonary or metabolic factors.
Ventricular pauses of >3 s, assessed by Holter monitoring, were more frequent with ticagrelor than with clopidogrel in the acute phase of the coronary event (during the first week), but not at 30 days after randomization. The risk of heart block, syncope and pacemaker implantation with ticagrelor was not significantly different from clopidogrel.
In addition to P2Y12 signal blockade, ticagrelor also inhibits equilibrative nucleoside transporter-1 (ENT-1), which is implicated in the cellular uptake of endogenous adenosine.16–18 The above adverse drug reactions may be related to this mechanism.
The Administration of Ticagrelor in the Cath Lab or in the Ambulance for New ST Elevation Myocardial Infarction to Open the Coronary Artery (ATLANTIC) trial assessed the potential benefits of the prehospital administration of ticagrelor compared with in-hospital administration.19 In this study involving 1862 STEMI patients, the median time from randomization to coronary angiogram was 48 min and the median time difference between pre-hospital and in-hospital administration of the ticagrelor loading dose was 31 min. The primary outcomes, surrogates of antithrombotic efficacy related to pre-PCI coronary reperfusion (resolution of ST-segment elevation and the proportion of coronary TIMI flow <3), were not improved by early pre-hospital ticagrelor administration. The risk of definite stent thrombosis was lower with pre-hospital ticagrelor administration.19
CangrelorCangrelor is an intravenous reversible P2Y12 inhibitor. The main advantages of this drug are rapid onset (within 2 min of a bolus followed by an infusion) and offset (one hour after stopping drug infusion) of antiplatelet effects.20
The drug is metabolized in the plasma into an inactive metabolite which is eliminated through renal and digestive routes. Thus, cangrelor does not require dose adjustments in renal or liver impairment.
Cangrelor has been approved for patients with coronary disease undergoing PCI who did not receive an oral P2Y12 inhibitor prior to the procedure and in whom current oral therapy with P2Y12 inhibitors is not feasible (e.g. absence of oral route) or desirable (e.g. bridging to CABG surgery).20
The pivotal CHAMPION PHOENIX (Cangrelor versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition PHOENIX) trial compared cangrelor and placebo in 10 942 patients treated with a 300- or 600-mg loading dose of clopidogrel before PCI.21 This trial showed an RRR of 22% and 15% in the composite endpoint of mortality and ischemic events (MI, ischemia-driven revascularization or stent thrombosis) at 48 hours and 30 days of follow-up, respectively. This benefit was mainly driven by a significant RRR of 38% in stent thrombosis (intraprocedural stent thrombosis independently adjudicated by the angiographic core laboratory committee, or definite stent thrombosis as defined by the Academic Research Consortium criteria22). CHAMPION PHOENIX included 1992 patients with STEMI and no interaction was observed regarding treatment effect according to clinical presentation (STEMI and non-STEMI).21
The benefits of cangrelor are likely related to its rapid antiplatelet action compared to other oral P2Y12 inhibitors such as clopidogrel.
Glycoprotein IIb/IIIa inhibitorsThis class includes three drugs, administered intravenously, that disable fibrinogen and von Willebrand factor by binding to the GP IIb/IIIa receptor: abciximab, tirofiban and eptifibatide.
AbciximabAbciximab is an antigen-binding fragment of a chimeric monoclonal antibody that binds to GP IIb/IIIa and vitronectin receptors. Abciximab's antiplatelet effect is seen 10 min after bolus administration and platelet function typically recovers 24-48 hours after drug withdrawal.23–25
Most of the evidence supporting the use of GP IIb/IIIa inhibitors in STEMI arises from trials with abciximab in the era of balloon angioplasty and before the routine implementation of dual antiplatelet therapy in these patients. De Luca et al. published a systematic review on the clinical impact of abciximab in STEMI patients treated with fibrinolysis or primary PCI.26 In this review abciximab reduced both 30-day and long-term (six months to one year) all-cause mortality by 31% and 32%, respectively. No significant increase in major bleeding risks, including intracranial hemorrhage, was noted (Figure 1).26
The main adverse event rather than bleeding (major or minor) is thrombocytopenia, which usually develops in the first 24 hours. The incidence of thrombocytopenia is 2.9% for first administration and 5% for repeat administrations.27,28
TirofibanTirofiban is a non-competitive non-peptide GP IIb/IIIa receptor antagonist. This drug's antiplatelet effect begins 15 min after a bolus administration of 0.25 μg/kg,29 and inhibition of platelet aggregation is <50% four hours after cessation of drug infusion.30
In the On-TIME 2 trial, 936 STEMI patients were randomized to high-dose tirofiban or placebo in the pre-hospital setting, on top of aspirin 500 mg, clopidogrel 600 mg, and unfractionated heparin 5000 IU.31 The trial was designed to assess ST-elevation resolution, and tirofiban significantly decreased the extent of residual ST-segment deviation one hour after PCI. The rates of the composite of death, recurrent MI or target vessel revascularization, and major bleeding were not significantly different between interventions. There was significantly less need for thrombotic bail-out due to TIMI flow grade 0-2 and abrupt closure of the culprit vessel in the tirofiban arm (Figure 1). In-hospital thrombocytopenia was not significantly higher (2.0% with tirofiban and 1.8% with placebo).
EptifibatideEptifibatide is a synthetic heptapeptide that reversibly and competitively inhibits GP IIb/IIIa receptors. It is given through an initial double intravenous bolus (separated by 10 min) of 180 μg/kg followed by a continuous infusion of 2 μg/kg/min for up to 72 hours. The antiplatelet effects are observed after the bolus and platelet function recovers four hours after cessation of infusion. Because 50% of eptifibatide clearance is kidney-dependent, the infusion dose should be halved in patients with moderate chronic kidney disease (estimated glomerular filtration rate 30-50 ml/min).
The main data on eptifibatide use in acute coronary syndromes come from the PURSUIT trial, which enrolled only patients without persistent ST-segment elevation.32 PURSUIT was a randomized, double-blind assessment of the efficacy and safety of eptifibatide versus placebo for reducing mortality and MI in patients with unstable angina or non-Q-wave MI and included 13.8% of patients with non-persistent ST segment elevation or persistent ST-segment elevation of 0.5-0.9 mm. This trial showed a RRR of 10% in mortality or MI at 30 days. A significant increase in major and/or minor bleeding events was observed in the eptifibatide arm. The risk of thrombocytopenia with eptifibatide was not significantly different from placebo.
In the context of STEMI, the Eptifibatide Versus Abciximab in Primary PCI for Acute Myocardial Infarction (EVA-AMI) trial randomized 427 STEMI patients to eptifibatide (double bolus and 24-hour infusion) or abciximab (bolus and 12-hour infusion) as adjuncts to primary PCI.33 The primary outcome was complete ST-segment resolution 60 min after PCI. The electrocardiographic and clinical impact of eptifibatide was similar to abciximab (Figure 1). Non-randomized studies show similar findings.34,35
HeparinsUnfractionated heparinUnfractionated heparin (UFH) is a polysaccharide that binds to plasma proteins, including antithrombin, enhancing its action, which includes inactivation of thrombin and factor Xa. The heparin-antithrombin complex also inhibits other coagulation factors including VIIa, XIa and XIIa. UFH also enhances endogenous fibrinolysis. UFH is usually given intravenously, including in the context of STEMI.
This drug has two independent clearance mechanisms: a saturable pathway in which rapid depolymerization follows binding to endothelial cells, macrophages and local proteins; and a non-saturable renal clearance pathway. The half-life of UFH is 90-120 min depending on the clearance mechanism. The existence of these two clearance mechanisms can lead to intra- and inter-individual variability in the pharmacodynamic effects of UFH, which is a limitation on its use due to the unpredictability of its anticoagulant effects, which necessitate monitoring by measurement of activated partial thromboplastin time or activated clotting time.
Although there have been no placebo-controlled trials, the value of UFH was highlighted by the OASIS-6 trial on fondaparinux, in which UFH was administered to some of the control group.36 In the subgroup of patients with STEMI who underwent primary PCI, UFH reduced the risk of thrombotic bailout or catheter thrombosis, none of the latter occurring with UFH. There was also a trend towards an increased risk of death or reinfarction in primary PCI patients treated with fondaparinux compared to UFH at 30 days and 90-180 days, with a significant subgroup interaction (primary PCI vs. non-primary PCI, trending for a favorable effect of UFH in the primary PCI subgroup, and a significant favorable effect of fondaparinux in the non-primary PCI subgroup).36 For these reasons fondaparinux is not recommended for patients with STEMI undergoing PCI, but is a valid anticoagulant for STEMI patients treated with fibrinolysis.3 UFH is an option for parenteral anticoagulants in this setting according to the European Society of Cardiology (ESC) guidelines.1
Regarding dosage of UFH, the initial bolus should be weight-adjusted, and should be as follows according to expectation of the use of GP IIb/IIIa inhibitors: UFH 70-100 IU/kg when no GP IIb/IIIa inhibitor is planned, or UFH 50-60 IU/kg when the use of GP IIb/IIIa inhibitors is expected.1
The main safety issues are bleeding events and thrombocytopenia. For bleeding events, protamine can be administered to reverse the anticoagulant effect of UFH. The incidence of heparin-induced thrombocytopenia (HIT) ranges from 0.1% to 5.0% and at least one quarter of these patients develop thrombotic complications. In HIT, the platelet count fall is 50% or more and the nadir is above 20×109/l.37 It usually occurs after 5-10 days of exposure to UFH or sooner if the patient was exposed in the previous 30 days.37 Both HIT and HIT-related thrombotic events are related to the development of immunoglobulins against complexes of platelet factor 4 and UFH.37
Low molecular weight heparinsLow molecular weight heparins (LMWH) are small antithrombin polysaccharides derived from UFH. LMWH have more anti-Xa than anti-IIa activity and enhance the release of tissue factor pathway inhibitors. These drugs do not bind to endothelial cells or macrophages, and hence have a predictable dose-effect relationship using bolus administrations. Their bioavailability is also higher, enabling effective subcutaneous administration. Regarding safety issues, the rate of HIT is lower with LMWH than with UFH, but in bleeding conditions the antidote protamine is less effective, since it only partially neutralizes the anti-Xa effect of LMWH. The new anti-factor Xa antidote andexanet alfa may be used to reverse enoxaparin's anticoagulant effect in patients requiring rapid hemostasis.38 However, the Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of FXA Inhibitors 4 (ANNEXA-4) trial only enrolled four patients with major bleeding treated with enoxaparin (out of 67 patients treated with factor Xa inhibitors).38
The main clinical evidence for the use of LMWH in STEMI patients derives from the Acute STEMI Treated with primary angioplasty and intravenous enoxaparin Or UFH to Lower ischemic and bleeding events at short- and Long-term follow-up study (ATOLL).39 Compared to UFH, enoxaparin (intravenous bolus of 0.5 mg/kg followed by subcutaneous injections every 12 hours) did not significantly (p=0.06) reduce the primary composite outcome of death, complication of MI, procedure failure, or major bleeding in 910 STEMI patients (Figure 2). However, secondary outcomes including death, recurrent acute coronary syndrome, and urgent revascularization were significantly decreased (41% RRR) with enoxaparin.39 No differences were observed in major bleeding. Based on these findings, the ESC guidelines state that LMWH may be considered instead of UFH (class IIb recommendation).1 Another advantage of LWMH over UFH is the lower risk of HIT.
Results of selected trials assessing anticoagulant agents in patients with ST-segment elevation myocardial infarction. CI: confidence interval; CV: cardiovascular; GP: glycoprotein; HR: hazard ratio; MI: myocardial infarction; OR: odds ratio; PCI: percutaneous coronary intervention; RR: relative risk; STEMI: ST-segment elevation myocardial infarction; UFH: unfractionated heparin.
Bivalirudin is a polypeptide that is a direct thrombin inhibitor, binding both circulating and clot-bound thrombin.
The Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial compared UFH plus a GP IIb/IIIa inhibitor with bivalirudin alone (0.75 mg/kg intravenous bolus followed by 1.75 mg/kg/hour infusion) in patients undergoing primary PCI.40
In 3602 STEMI patients undergoing primary PCI, bivalirudin, compared to UFH plus GP IIb/IIIa inhibitors (52% abciximab, 46% tirofiban), showed an RRR of 24% within 30 days in net adverse clinical events, a composite of cardiovascular death, MI, target vessel revascularization, stroke or major bleeding, mostly due to the 40% RRR in major bleeding (Figure 2).40 An increased risk of stent thrombosis within 24 hours was observed in the bivalirudin group.
The European Ambulance Acute Coronary Syndrome Angiography (EUROMAX) trial studied 2218 STEMI patients transported for primary PCI and randomized to bivalirudin (bolus and infusion for at least four hours after PCI at 0.25 mg/kg/hour) or UFH (and optional use of GP IIb/IIIa inhibitors).41 The primary outcome of death and major bleeding was significantly decreased, due to the 57% RRR in major bleeding.41 Similarly to the HORIZONS-AMI trial, the risk of acute stent thrombosis was significantly higher.
The Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox (MATRIX) trial also compared bivalirudin with UFH (with GP IIb/IIIa inhibitors only in bail-out circumstances).42 This trial included 7213 ACS patients, 4010 (56%) with STEMI. The results for the STEMI group were not different from the overall study results.43 The risk of death, non-fatal MI and non-fatal stroke was similar between groups, but there was a significant 40% RRR in major bleeding with bivalirudin in the STEMI group (Figure 2). The MATRIX study also assessed the potential impact of post-PCI bivalirudin infusion; this strategy did not improve any outcome, including stent thrombosis.42
The Bivalirudin versus Heparin in ST-Segment and Non-ST-Segment Elevation Myocardial Infarction in Patients on Modern Antiplatelet Therapy in the Swedish Web System for Enhancement and Development of Evidence-based Care in Heart Disease Evaluated according to Recommended Therapies Registry Trial (VALIDATE-SWEDEHEART) was a randomized open-label registry-based controlled clinical trial that compared bivalirudin and UFH monotherapy in 6006 patients with MI (3001 with STEMI). The clinical impact of bivalirudin was similar to UFH in monotherapy in the overall cohort as well as in the STEMI subgroup in terms of the composite primary endpoint of all-cause mortality, MI or major bleeding during 180 days of follow-up (Figure 2). No significant differences were observed in any of the secondary endpoints.
DiscussionSTEMI is an acute thrombus-driven event. The importance of this pathophysiological link is highlighted in this review, in which the main pharmacokinetic, pharmacodynamic and clinical data on antithrombotic drugs in STEMI are reviewed. These adjuvant medical agents, particularly antiplatelet drugs, have significantly improved the prognosis of this patient group. After aspirin (with ISIS-2),6 the breakthrough of P2Y12 inhibitors with ticlopidine marked a historic step. In the STARS trial the combination of ticlopidine and aspirin in stented patients (patients with recent MI were excluded) showed a decrease in stent thrombosis-related events compared to aspirin alone and aspirin combined with warfarin.44 In the ISAR trial the combination of aspirin and ticlopidine (24% of the patients in this group had had acute MI) was superior to aspirin with phenprocoumon (a vitamin K antagonist).45 The former reduced the composite endpoint of cardiovascular mortality, non-fatal MI or revascularization, as well as thrombotic stent occlusion.45 Consequently, the advent of GP IIb/IIIa inhibitors contributed significantly to the perception that dual antiplatelet therapy was necessary in acute coronary syndromes. The process of PCI, including intervention in STEMI and the evolution to drug-eluting stents and radial access, and inhibition of the P2Y12 receptor pathway with clopidogrel, prasugrel and ticagrelor, have since been further studied and optimized. Nowadays the mainstay for antiplatelet therapy in STEMI is aspirin and a potent P2Y12 inhibitor (usually ticagrelor or prasugrel). GP IIb/IIIa inhibitors are reserved for bailout situations, while cangrelor may play a role due to its rapid antiplatelet action in cases where the pharmacodynamic effects of the newer and faster P2Y12 inhibitors are not optimal. In STEMI, the fibrin-rich thrombi that occlude arteries are the result of platelet activation and also of the release of tissue factor by the damaged vessel that leads to activation, amplification and propagation of the coagulation cascade. Anticoagulants in this setting are given to prevent expansion and enhancement of thrombi. Furthermore, contemporary management with primary PCI requires a suitable antithrombotic environment to prevent catheter thrombosis, which may be achieved with UFH, enoxaparin or bivalirudin. Fondaparinux was found to decrease the risk of death or MI, particularly in patients managed medically or in those receiving fibrinolysis (mostly streptokinase). In patients undergoing primary PCI, fondaparinux increased the risk of catheter thrombosis and no clinical benefit was noted regarding other outcomes. Fondaparinux is therefore contraindicated in patients undergoing primary PCI. Regarding the other anticoagulants, UFH remains the gold standard intravenous anticoagulant, although its anti-ischemic efficacy was challenged by enoxaparin in one trial (ATOLL39), and despite concerns of increased risk of stent thrombosis the major bleeding risk was shown to be lower with bivalirudin.
The 2017 European Society of Cardiology recommendations for antithrombotic therapy in patients with ST-elevation myocardial infarction undergoing primary percutaneous intervention3Antiplatelet drugs- -
Aspirin is recommended (class I).
- -
A P2Y12 inhibitor is recommended in addition to aspirin (class I), particularly ticagrelor (class I) or prasugrel (in clopidogrel-naïve patients aged <75 years or with no history of stroke or transient ischemic attack – class I).
- -
GP IIb/IIIa inhibitors should be considered for bailout therapy in patients with high thrombotic burden or complications, including slow flow or no-reflow (class IIa).
- -
GP IIb/IIIa inhibitors may be considered for upstream therapy in high-risk patients undergoing transfer for primary PCI (class IIb), or as routine adjunct antiplatelet therapy in patients treated with UFH (class IIb).
- -
Bivalirudin is recommended over routine UFH with GP IIb/IIIa inhibitors (class I).
- -
Enoxaparin may be preferred to UFH (class IIa).
- -
UFH should be used in STEMI patients undergoing primary PCI not receiving bivalirudin or enoxaparin (class I).
- -
Fondaparinux is contraindicated in STEMI patients undergoing primary PCI (class III).
Table 1 summarizes the recommendations and their classes regarding antithrombotic therapy in patients with STEMI treated with primary PCI.
2017 European Society of Cardiology recommendations for antithrombotic therapy in patients with ST-elevation myocardial infarction undergoing primary percutaneous intervention.3
| Class of recommendation (wording) | I(Is recommended) | IIa(Should be considered) | IIb(May be considered) | III(Is not recommended) |
|---|---|---|---|---|
| Antiplatelet agents | Oral or IV aspirinP2Y12 inhibitor in addition to aspirinTicagrelorPrasugrel (clopidogrel-naïve patients aged <75 years; no history of stroke or TIA)Clopidogrel when ticagrelor or prasugrel are unavailable or contraindicated | GP IIb/IIIa inhibitors as bailout therapy in patients with high thrombotic burden, slow flow or no-reflow | GP IIb/IIIa inhibitors as upstream therapy in high-risk patients transferred for primary PCIRoutine use of GP IIb/IIIa inhibitors in addition to UFH in primary PCI | - |
| Anticoagulant agents | Bivalirudin over routine UFH with GP IIb/IIIa inhibitorsUFH in primary PCI patients not receiving bivalirudin or enoxaparin | - | Enoxaparin over UFH | Fondaparinux in primary PCI patients |
GP: glycoprotein; IV: intravenous; PCI: percutaneous coronary intervention; TIA: transient ischemic attack; UFH: unfractionated heparin.
Advances in antithrombotic treatment have led to significant improvements in the prognosis of patients with STEMI treated by primary PCI. The growing body of evidence on each of these therapeutic options poses new challenges in terms of drug combinations.
Conflicts of interestThe authors have no conflicts of interest to declare.