Systemic sclerosis (SSc) is a systemic autoimmune disease involving multiple organs. We present a rare case of SSc in which clinical manifestations of cardiac fibrosis occurred early in the disease course.
Case reportWe report the case of a 40-year-old Caucasian man, previously diagnosed with SSc, who presented with decompensated heart failure. Transthoracic echocardiography was remarkable for severe right ventricular systolic dysfunction, abnormal ventricular septal motion, severe functional tricuspid regurgitation and normal pulmonary artery systolic pressure. Left ventricular ejection fraction was 45%. Right heart catheterization revealed no signs of pulmonary hypertension. Cardiac magnetic resonance (CMR) showed diffuse myocardial infiltration, later confirmed as myocardial fibrosis by endomyocardial biopsy.
ConclusionsMyocardial fibrosis is an important cause of early heart failure in SSc patients and is associated with poor prognosis. Echocardiography and CMR help establish the diagnosis and enable an appropriate therapeutic strategy to be developed in such cases.
A esclerose sistémica (ES) é uma doença autoimune sistémica que pode envolver múltiplos órgãos. Apresentamos um caso raro de ES associada a manifestação clínica de fibrose miocárdica no decurso precoce da evolução da doença.
Caso clínico: Reportamos um caso de um homem de 40 anos, com antecedentes de ES e que se apresenta com um quadro de insuficiência cardíaca descompensada. O ecocardiograma transtorácico revelou disfunção sistólica severa do ventrículo direito, movimento anómalo do septo interventricular, insuficiência tricúspide severa e pressão sistólica da artéria pulmonar normal. A fração da ejeção era de 45%. O cateterismo direito não identificou sinais de hipertensão pulmonar. A ressonância magnética cardíaca (RMC) demonstrou infiltração miocárdica difusa, confirmada com biópsia endomiocárdica como fibrose miocárdica.
ConclusõesA fibrose miocárdica é uma causa importante de insuficiência cardíaca na ES e está associada a mau prognóstico. O ecocardiograma e a RMC podem ajudar a estabelecer o diagnóstico e a desenvolver a estratégia terapêutica adequada.
Systemic sclerosis (SSc) is a chronic systemic autoimmune disease characterized by diffuse microvascular damage and fibrosis involving multiple organs.1 Although cardiac involvement may be detected early in the disease course, clinical manifestations are a late finding and are associated with poor prognosis.1,2 The prevalence of cardiovascular symptoms ranges between 10% and 35%.3–5
Cardiac involvement can be primary, affecting all layers of the heart (endocardium, myocardium and pericardium), or secondary to the impairment of other organs, such as the lungs and kidneys.1,2 All cardiac structures may be involved, leading to conduction system defects, atrial and ventricular arrhythmias, valvular defects, myocardial ischemia and/or hypertrophy, pericardial effusion or constrictive pericarditis.1,2,6,7 Heart failure may develop as a consequence of diffuse myocardial fibrosis or secondary to pulmonary hypertension.6 Diffuse myocardial fibrosis develops long before the onset of heart failure symptoms.1,3 Vascular lesions in SSc result in general impairment of the microcirculation, which in turn is associated with abnormal vasoreactivity of the small vessels. This process can produce repeated focal ischemia, eventually leading to fibrosis.1,3
Several imaging methods can be used to detect cardiac involvement in SSc, including echocardiography and myocardial perfusion scanning. However, both lack sensitivity for certain tissue characteristics, particularly inflammation and fibrosis, which can be accurately identified by cardiac magnetic resonance (CMR).6,8
Here, we present a rare case of a patient recently diagnosed with SSc admitted with heart failure, which was ultimately found to be associated with diffuse and severe intramyocardial fibrosis.
Case reportA 40-year-old Caucasian man presented to the emergency room with asthenia, dyspnea on mild exertion and peripheral edema. The symptoms had started three weeks previously and had been worsening progressively. He also had complaints suggestive of Raynaud phenomenon and hardening of the skin in the previous eight months.
Two years before this episode, following an abrupt onset of peripheral edema in both hands and lower limbs, he had been diagnosed with SSc. At the time, immunological tests were performed, which were positive for anti-Scl-70 antibodies. He also underwent lung function tests, which were normal except for mildly reduced carbon monoxide diffusion capacity, and high-resolution chest tomography, which was negative for pulmonary fibrosis. Since then he had been treated with mycophenolate mofetil, prednisolone and monthly cyclophosphamide pulses.
On admission, physical examination revealed hardened skin on the hands, forearms, legs, feet and abdominal wall (Rodnan skin score >20). Raynaud phenomenon was also present on both hands and feet. Lung auscultation demonstrated slight rales in both pulmonary bases. Signs of overt right heart failure were also present, including increased jugular venous pressure of 16 cm H2O, Kussmaul's sign, ascites and lower limb edema.
Laboratory workup was notable for markedly elevated brain natriuretic peptide (BNP) of 2340 pg/ml (normal <100 pg/ml) and hepatic congestion, with alkaline phosphatase (ALP) of 467 U/l (normal <120 U/l) and gamma-glutamyl transpeptidase (GGT) of 367 U/l (normal <45 U/l). The admission electrocardiogram showed sinus rhythm with right axis deviation, with no signs of right or left ventricular (LV) hypertrophy. Transthoracic echocardiography revealed severe right ventricular (RV) systolic dysfunction (tricuspid annular plane excursion [TAPSE] 13 mm, S’ 6 cm/s, fractional area change [FAC] 24%, RV ejection fraction [RVEF] 39%), paradoxical ventricular septal motion suggestive of volume/pressure overload, severe tricuspid regurgitation and pulmonary artery systolic pressure (PASP) of 16 mmHg (likely underestimated). There was also mild LV systolic dysfunction (LV ejection fraction [LVEF] 45% estimated by the Simpson biplane method), impaired global longitudinal strain (GLS) -8%, type 2 diastolic dysfunction with elevated filling pressures (E/E’ ratio 15), and moderate mitral regurgitation.
Due to suboptimal clinical response to initial diuretic therapy, the patient was transferred to an advanced heart failure unit to further optimize treatment, with intensive diuretic therapy and vasopressor support with noradrenaline. During admission, the patient suffered two cardiopulmonary arrests in ventricular fibrillation. In both episodes, there was full neurologic recovery after advanced cardiac life support and prompt defibrillation. Coronary angiography showed no coronary artery disease.
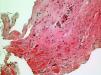
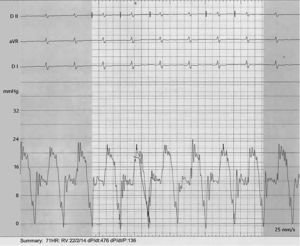
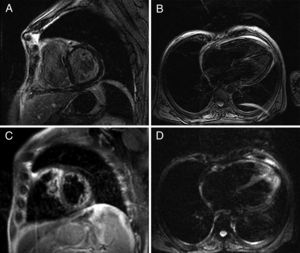
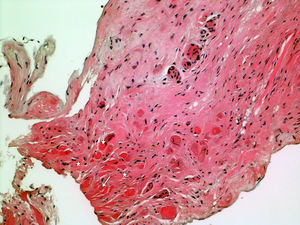
In order to better understand the hemodynamics, right heart catheterization was performed and revealed poor RV function with high filling pressures, normal pulmonary artery pressures, mildly depressed cardiac index, normal pulmonary capillary wedge pressure and normal pulmonary vascular resistance (Table 1). Importantly, a deep y descent with a rapid upstroke was noted (‘square root’ sign), suggesting severe diastolic filling impairment (Figure 1). To further clarify the myocardial disorder in the absence of pulmonary hypertension, CMR was performed, which showed diffuse infiltration of the myocardium (Figure 2). This was later confirmed by endomyocardial biopsy which was notable for diffuse myocardial fibrosis (Figure 3).
Right heart catheterization.
| Variable | |
|---|---|
| Aorta (S/D/M) | 104/57/72 mmHg |
| PA (S/D/M) | 22/11/16 mmHg |
| RV (S/BD/ED) | 22/2/14 mmHg |
| RA (M) | 16 mmHg |
| PCWP (M) | 10 mmHg |
| CO | 3.86 l/min-1 |
| CI | 2.12 l/min/m2 |
| PVR | 1.6 UW |
BD: beginning diastolic; CI: cardiac index; CO: cardiac output; D: diastolic; ED: end-diastolic; M: mean; PA: pulmonary artery; PCWP: pulmonary capillary wedge pressure; PVR: pulmonary vascular resistance; RA: right atrium; RV: right ventricle; S: systolic.
Due to the patient's susceptibility to arrhythmias, an implantable cardioverter-defibrillator (ICD) was placed. A significant clinical response to intensive decongestive therapy was achieved. At discharge, a total net weight loss of 15 kg was recorded. Combined treatment for heart failure and SSc was instituted, including intravenous immunoglobulin G (400 mg/kg/day) initially for five consecutive continuous days and then monthly, and, in a second phase, rituximab.
At six-month follow-up, the patient had had one appropriate ICD shock. Echocardiography showed an improved LVEF of 56% and GLS of -15%, with continuing type 2 diastolic dysfunction, and depressed but improved RV function (TAPSE 14 mm, S’ 7 cm/s, FAC 31%, RVEF 40%). Laboratory workup revealed reduced BNP (350 pg/ml), normal transaminases and ALP, and elevated GGT of 300 U/l. No other admissions to the emergency department, episodes of decompensated heart failure or arrhythmic events were recorded at 14 months of follow-up.
DiscussionWe present a rare case of SSc with clinically overt right heart failure due to severe fibrotic involvement of the myocardium, occurring early in the disease course. While diffuse myocardial fibrosis is known to be present histologically early in SSc, it is usually clinically silent until the late stages of the disease.1–3
In several studies, a reduced LVEF was found in only a minority of SSc patients, ranging from 1.4% to 5.4%.7,8 However, a study of 100 patients using a more sensitive echocardiographic technique to assess systolic function based on tissue Doppler demonstrated that 14% and 15% of patients with SSc had impaired LV and RV contractility, respectively. Moreover, this study found that 30% of patients had definite abnormal LV filling patterns.9 It can thus be concluded that diastolic dysfunction is more prevalent than systolic dysfunction in both ventricles in SSc patients. Another study investigated cardiac function in 46 SSc patients with normal pulmonary arterial pressures and less than five years of disease.10 The authors found that 16 patients had reduced RVEF and 10 had reduced peak filling rate, demonstrating early right systolic and left diastolic dysfunction. Nevertheless, no correlation was found with pulmonary function impairment or pulmonary arterial pressure.10 This type of cardiac involvement differs from that caused by pulmonary arterial hypertension, the prevalence of which ranges from 8% to 12%.11,12
Our patient had mildly reduced LVEF (45%) and severely impaired RVEF, with paradoxical ventricular septal motion but normal PASP. The congestive symptoms were attributed to the early impairment of RV systolic and LV systolic and diastolic dysfunction; notably, RV dysfunction appeared without the development of pulmonary vascular disease, a much more obvious clinical correlation in SSc, and was solely the consequence of the myocardial fibrotic process.
CMR is currently the noninvasive method of choice to assess diffuse myocarditis, providing information on the stage, degree, and extent of reversible and irreversible myocardial injury.13 T1- and T2-weighted CMR are novel techniques for quantitative tissue characterization, which may overcome the limitations of current CMR criteria for assessing diffuse myocardial damage.13,14 Recent studies indicate that native myocardial T1, myocardial T2, and extracellular volume (ECV) imaging could improve the diagnostic performance of CMR.14,15
The therapeutic strategy was changed in the case presented to a more aggressive off-label immunosuppressive treatment. There is in fact no evidence that intravenous immunoglobulin G and rituximab are more effective than other immunosuppressant drugs, though some recent studies support their use in SSc patients, particularly those with severe skin and lung involvement.16,17
Unfortunately, no immunomodulatory strategy has yet been shown to specifically reduce myocardial fibrosis. Although there is no formal contraindication to heart transplantation, it is usually not performed in these cases, since the transplanted heart may be involved by the same infiltrative process.
ConclusionsWhen approaching SSc patients presenting with heart failure it is important to recognize that pulmonary hypertension is not the cause in all cases, and to bear in mind other possible forms of cardiac involvement in this disease. Early detection of cardiac involvement, including diffuse myocardial fibrosis, is essential in SSc patients, since it is associated with poor prognosis. Use of accurate diagnostic tools such as echocardiography and CMR help establish the cause of heart failure and enable initiation of an appropriate therapeutic strategy.
ConsentWritten informed consent was obtained from the patient for publication of this case report and any accompanying images.
Authors’ contributionsPA: conception and design, analysis and interpretation of data, manuscript writing. RB, CC, FF: attending physician, acquisition and analysis of data, critical revision for important intellectual content. LS and MP: interpretation of data, critical revision for important intellectual content. All authors read and approved the final manuscript.
Availability of data and materialsSupporting data is available to researchers in a repository.
Conflicts of interestsThe authors have no conflicts of interest to declare.