We present a case of post-infarction papillary muscle rupture occurring after successful reperfusion of an occluded left circumflex artery. The diagnosis of this increasingly rare complication was made possible by prompt bedside echocardiographic assessment after clinical deterioration. Pathology findings corroborated imaging data, clarifying the relation between irreversible structural disruption and functional consequences in this setting.
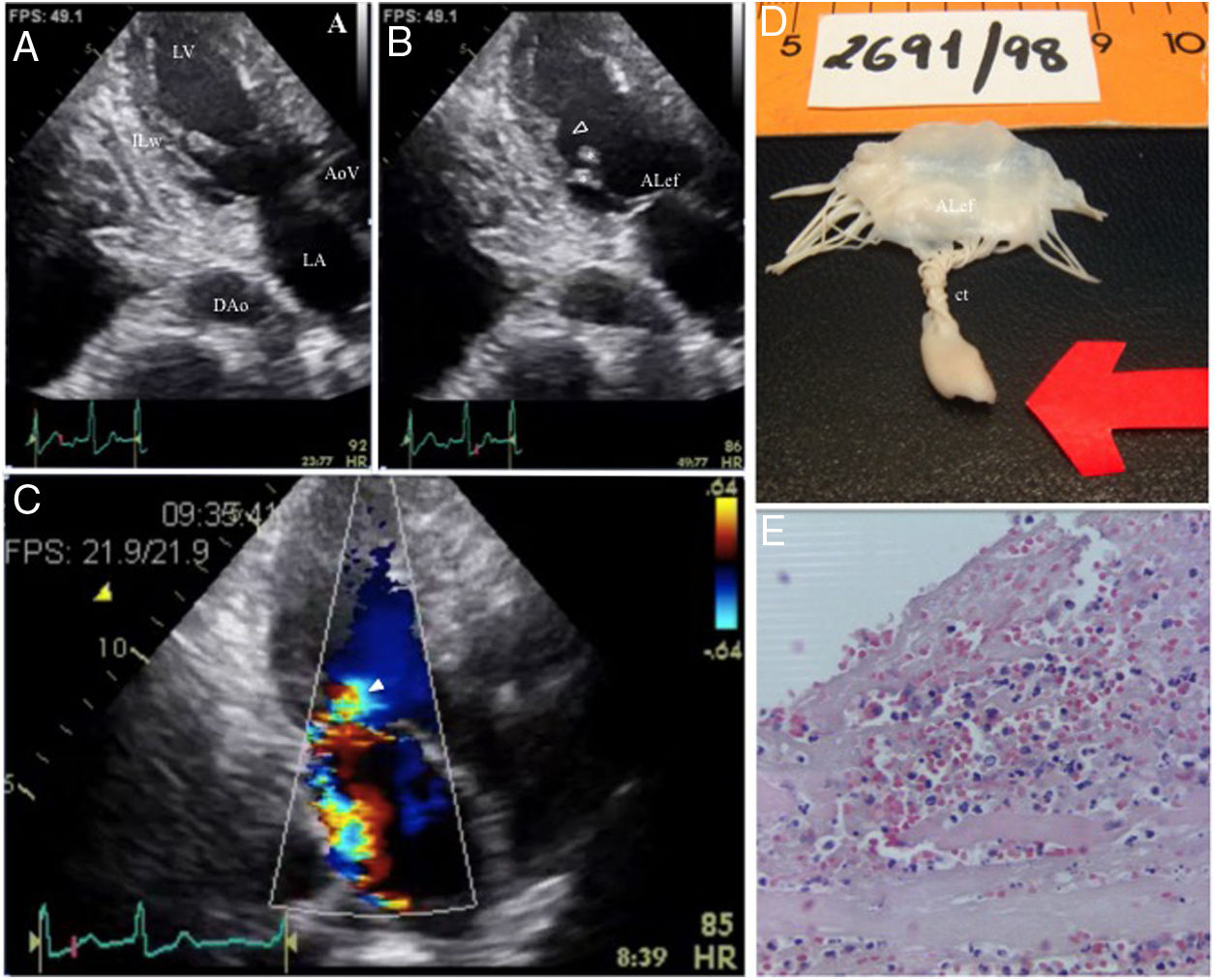
We describe the case of a 76-year-old overweight and hypertensive female patient admitted to the emergency department because of persistent chest pain at rest starting in the previous four hours. Her 12-lead electrocardiogram showed ST-segment elevation in the DI, aVL and V5-V6 precordial leads. Coronary angiography was performed after transfer to a tertiary hospital within 40 min of admission. It revealed left coronary artery dominance and thrombotic occlusion of the left circumflex artery, for which thrombus aspiration and stent implantation were performed. Echocardiographic assessment showed mildly impaired left ventricular ejection fraction with regional akinesis in both lateral and inferolateral left ventricular wall segments. Sudden pulmonary edema with hypotension 24 hours after admission prompted echocardiographic reassessment, which was notable for posteromedial papillary muscle rupture with severe eccentric mitral valve regurgitation. A Coanda effect across the left atrial posterior wall with anterior leaflet bending and prolapse, and a single color jet in 3-chamber apical view, were in favor of exclusive anterior leaflet involvement (predominant A2 involvement without posterior leaflet involvement) (Figure 1A–C). A hypermobile papillary muscle with disrupted tip and concurrent significant regurgitation at qualitative color Doppler interrogation (flow convergence without baseline adjustment) provided the diagnosis, with no need for additional quantitative parameters supporting severity.
Echocardiographic and pathologic findings. Four proposed criteria for papillary muscle rupture (B: arrowhead, D: arrow) are present: (1) mobile masses (*) with erratic motion inside the left ventricle, (2) prolapse and bending of anterior mitral valve leaflet (ALef), (3) mitral regurgitation (C: arrow), and (4) ventricular wall motion abnormality with inferolateral wall (ILw) akinesia, not resulting in significant reduction in left ventricular cavity dimensions in systole (B); D: gross pathology; E: microscopic findings (HE ×400). AoV: aortic valve; ct: chordae tendineae; DAo: descending aorta; HE: hematoxylin-eosin; LA: left atrium; LV: left ventricle.
Intra-aortic balloon pump and inotropic drugs were instituted and the patient was referred for surgical mitral valve intervention. Mitral valve replacement was successfully accomplished with an uneventful recovery. Except for the necrosed and ruptured papillary muscle tip, the submitral apparatus was left in place.
Gross pathologic findings were remarkable, showing the infarcted, ruptured papillary muscle head, irregular borders (z-shaped) at the point of rupture, and interwoven and shrunken free chordae tendineae, explaining the freely mobile margins of the anterior mitral leaflet (Figure 1D). Microscopic examination (Figure 1E) revealed coagulation necrosis, neutrophil infiltration, cell debris, hemorrhage and fibrin deposition at the papillary muscle rupture zone (hematoxylin-eosin ×400). This was consistent with irreversible tissue damage, which explains the papillary muscle tip disruption, leaflet eversion and functional consequences with severe regurgitation at echocardiographic assessment.
The posteromedial muscle usually receives its blood supply exclusively from the right coronary artery, except when there is left coronary dominance. Post-myocardial infarction papillary muscle rupture is a rare and unexpected complication in the current era of coronary reperfusion, particularly when this is performed in due time, as in this case. Nevertheless, its outcome is extremely adverse, with high mortality if it is not immediately recognized and is left under medical therapy. Preservation of the subvalvular apparatus, lower preoperative risk and absence of inotropic drug support are strong independent predictors of better overall long-term survival after surgery.
Echocardiography at the bedside enables rapid and accurate diagnosis of post-myocardial infarction mechanical complications, with earlier surgical referral. Nonetheless, correlation with pathology still clarifies the mechanisms of disease and organic valve dysfunction in this setting.
Authors’ contributionsMC, JA, RR and RG were responsible for the original idea, clinical data collection and manuscript writing; MA performed the surgical intervention; RG performed the pathologic interpretation. All the authors reviewed and approved the final manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.