Increased fructose consumption is associated with various metabolic changes that favor the onset of obesity and related comorbidities. The objective of this study was to assess the effects of chronic fructose consumption on body weight and adipose tissue, as well as on serum glucose and triglyceride levels.
MethodsThirty-day-old Wistar rats were divided into two groups: fructose (F) and control (C), which had free access to commercial chow and either water or a 20% fructose solution. Body mass was measured weekly and food consumption at 30, 60 and 90 days. At 90 days, the animals were killed by decapitation and fat deposits (mesenteric, epididymal and retroperitoneal) were removed and blood collected for measurement of glucose and triglyceride levels.
ResultsThere was no significant difference in body weight gain, but the percentage of body fat was higher in group F. This group also consumed less feed at 60 and 90 days and had higher consumption of fructose solution than water in group C at 30 and 60 days. This meant higher calorie intake in group F and lower feed efficiency. Retroperitoneal and epididymal fat deposits and triglycerides were higher in group F than in group C.
ConclusionConsumption of fructose solution for eight weeks, while not directly reflected in body weight gain, did increase abdominal fat in group F compared to group C, as well as changing triglyceride levels. These two factors increase risk of cardiovascular disease.
O aumento do consumo de frutose pela população vem sendo associado às diversas alterações metabólicas, que favorecem ao aparecimento da obesidade e suas comorbidades. O objetivo deste trabalho foi avaliar os efeitos do consumo crônico de frutose sobre o consumo alimentar, ganho de massa corporal e de tecido adiposo, além de níveis séricos de glicose e triglicerídeos.
MétodosRatos Wistar com 30 dias de vida foram divididas em dois grupos: frutose (F) e controle (C), os quais receberam um tratamento com livre acesso a ração comercial, água ou solução a 20% de frutose. A massa corporal foi avaliada semanalmente e o consumo alimentar aos 30, 60 e 90 dias. Aos 90 dias de vida, os animais foram eutanasiados por decapitação e retirados os depósitos de gordura (mesentérico, retroperitoneal e epididimal), e coletado o sangue para dosagem da glicose e triglicerídeos.
ResultadosNão houve diferença significativa entre o ganho de massa corporal, todavia os percentuais de gordura corporal foram maiores nos grupos que consumiram bebidas adoçadas. O grupo F consumiu menor quantidade de ração aos 60 e 90 dias e maior consumo de solução de frutose comparado ao controle hídrico nos períodos de 30 e 60 dias, e isso significou maior consumo calórico do grupo F e menor eficiência alimentar. Os depósitos de gordura retroperitoneal e epididimal, bem como a trigliceridemia apresentaram-se elevados no grupo F quando comparado ao grupo C.
ConclusãoO tratamento por 60 dias com solução de frutose, apesar de não ter influenciado diretamente no ganho de massa corporal, foi capaz de aumentar a gordura corporal na região abdominal neste grupo, quando comparado com o grupo C, além de alterar níveis de triglicerídeos. E estes dois fatores implicam risco de doenças cardiovasculares.
Diet plays an important role in the development of cardiovascular disease (CVD), and sugar intake appears to be linked to its risk factors.1 Studies have shown the health risks associated with fructose consumption, in both humans2 and rodents,3 whether consumed in pure form or in combinations of carbohydrates, and at different concentrations and durations of ingestion.
Fructose, or levulose, is a monosaccharide, composed of six carbon atoms in simple covalent bonds, with a carbonyl group at the end of the chain (molecular formula C6H12O6), and is a ketohexose. It is found in the diet in corn syrup, sucrose, soft drinks, and fruit and fruit-derived products. Its consumption in the form of soft drinks is a public health concern.4,5
Although fructose has the same molecular formula and mass as glucose, physiologically they are processed differently. Fructose does not require insulin for cellular internalization. Phosphorylation of fructose into fructose-1-phosphate occurs through the action of fructokinase, leading to the formation of two pentoses, dihydroxyacetone and glyceraldehyde. This enzyme is not regulatory and can thus stimulate lipogenesis. Similarly, glucose is phosphorylated twice before entering the fructose pathway, in which it is also broken down into dihydroxyacetone and glyceraldehyde, but its metabolism depends on a limiting enzyme, phosphofructokinase, which is inhibited by ATP and citrate, which limits the accumulation of carbon-rich intermediaries entering the Krebs cycle.6
Among the metabolic disorders associated with fructose are hypertriglyceridemia, hyperuricemia, hyperinsulinemia, hepatic steatosis, vascular compromise, peripheral insulin resistance and increased visceral adiposity, and there may also be increased food intake and body mass and obesity.5
These effects have sparked interest in the role of fructose consumption in the development of the metabolic syndrome and CVD, and it is thus important to understand the mechanisms involved in fructose metabolism. The objective of this study was to assess the effects of chronic fructose consumption on food intake, body weight and adipose tissue, as well as on serum glucose and triglyceride levels.
MethodsStudy designAll experimental procedures were approved by the research ethics committee of the Federal University of Rio de Janeiro. Thirty-day-old male Wistar rats weighing around 85 g from a local laboratory animal supplier, were divided into a control group (C) (n=10) and a fructose group (F) (n=10), and had free access to commercial chow and water (group C) or 20% fructose solution (group F) for 60 days, under a 12-h light cycle (6 am to 6 pm) at a constant temperature (24±1°C).
Food intakeTo assess food intake, the rats were kept in individual cages and consumption was calculated as the difference between the amount of chow and fructose or water provided and the amount remaining in a 24-h period at 30 days (beginning of treatment), 60 days (half-way point) and 90 days (end of treatment). Energy intake was estimated on the basis of 9 kcal/g for lipids and 4 kcal/g for protein and carbohydrate from chow and 20% fructose solution. Data were expressed per 100 g of body weight.
Feed efficiency was estimated using the following formula: (body weight gain (g)/energy intake (kcal))×100, adapted from Novelli et al.,7 and the results were presented in percentages.
Body mass and fat depositsBody mass was assessed weekly from the beginning of treatment to determine weight gain in each group.
Epididymal, mesenteric and retroperitoneal fat deposits were assessed by gravimetry, total adipose tissue being taken as the sum of the values for the above three compartments. The results were expressed in g of fat per 100 g of carcass.
Glycemia and triglyceridemiaAfter 12 h fasting, the animals were killed by decapitation and their blood was collected and centrifuged at 2500 rpm for 15 min at 4°C. The serum was then used to measure glucose by the enzymatic method using a commercial kit (Glucose PAP Liquiform, Labtest Diagnostics, Minas Gerais, Brazil). Triglycerides were measured by the colorimetric method using a commercial kit (Triglycerides Liquiform, Labtest Diagnostics, Minas Gerais, Brazil). Both assays used an absorbance of 505 nm.
Statistical analysisThe results were expressed as means ± standard error of the mean and the Student's t test was used in IBM SPSS software, version 20.0. A level of significance of p<0.05 was adopted.
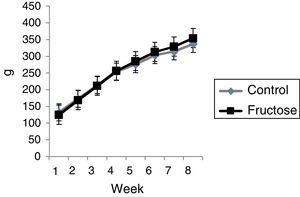
ResultsThe two groups had similar body mass at the beginning of treatment, and after 60 days, there was no significant difference between the groups in either weekly weight gain (Figure 1) or final weight gain (Table 1). However, group F presented greater total adipose tissue, as well as significantly higher percentages of epididymal and retroperitoneal adipose tissue; they also had a non-significant 38% increase in mesenteric adipose tissue compared to group C (Table 1).
Body mass, adipose tissue and serum glucose and triglyceride levels.
| Control group | Fructose group | |
|---|---|---|
| Body weight gain (g) (n=10) | 253±5.21 | 269.7±9.12 |
| Retroperitoneal adipose tissue (g/100 g) (n=9) | 0.58±0.06 | 0.94±0.07* |
| Mesenteric adipose tissue (g/100 g) (n=9) | 0.67±0.08 | 0.87±0.08 |
| Epididymal adipose tissue (g/100 g) (n=9) | 0.74±0.06 | 0.97±0.06* |
| Total adipose tissue (g/100 g) (n=9) | 1.99±0.06 | 2.78±0.18* |
| Glycemia (mg/dl) (n=9) | 96.2±3.71 | 104.7±5.53 |
| Triglyceridemia (mg/dl) (n=10) | 130.5±5.56 | 162.32±12.31* |
With regard to food intake, group F presented lower chow intake at 60 and 90 days, while total energy intake and fructose solution consumption were higher at 30 and 60 days; feed efficiency was lower in group F during the latter two periods (Table 2).
Food intake and feed efficiency.
| Parameters | Days of life | |||||
|---|---|---|---|---|---|---|
| Control group | Fructose group | |||||
| 30 days | 60 days | 90 days | 30 days | 60 days | 90 days | |
| Food intake (g/100 g) (n=9/8) | 15.18±0.67 | 9.88±0.47 | 9.20±0.31 | 13.66±0.72 | 7.09±0.29* | 5.38±0.22* |
| Energy intake (kcal/100 g) (n=9/8) | 63.76±2.82 | 41.48±1.96 | 38.64±1.30 | 85.81±3.90* | 47.32±1.35* | 41.54±1.46 |
| Water/fructose solution consumption (ml/100 g) (n=9/8) | 28.32±2.34 | 17.64±0.40 | 19.91±0.81 | 35.56±2.23* | 21.90±1.22* | 23.64±1.88 |
| Feed efficiency (%) (n=9/8) | 77±0.01 | 315±0.12 | 220±0.11 | 47±0.03* | 272±0.14* | 230±0.09 |
There was no significant difference in serum glucose levels between the two groups, but triglyceride levels were higher in group F (Table 1).
DiscussionThe present study demonstrated that consumption of 20% fructose solution in Wistar rats leads to gains in body mass and adipose tissue, as well as changes in serum glucose and triglyceride levels.
Fructose appears to interfere with energy homeostasis, although it does not directly affect food intake. In any event, the significance of changes in these parameters is the subject of debate and our study found no difference between the two groups. This indicates that the metabolism of fructose is inefficient and does not in fact contribute to energy intake or result in body weight gain, even though this might be expected since fructose, as a carbohydrate, provides 4 kcal/g.4
This corroborates a 1995 study by Dai and McNeill8 who found no increase in body mass with increasing consumption of fructose, even after 14 weeks. However, other studies using 10% and 20% fructose solutions have found a positive association between chronic ingestion of this monosaccharide and body weight gain in hamsters.9,10
Although our study did not find changes in body mass, it has been reported that fructose disrupts the complex mechanisms that maintain energy intake and energy expenditure in balance.11 Furthermore, we showed that total fat mass and epididymal and retroperitoneal deposits were significantly greater in group F, demonstrating that pure fructose may contribute to adipogenesis.
Interestingly, some studies indicate that fructose ingestion promotes greater accumulation of body fat in mice than water and other types of sweetened beverages,9 as well as increasing adipocyte size12 and visceral adiposity.13 In rats, retroperitoneal and epididymal fat correspond in humans to central and visceral adipose tissue, respectively,14 and visceral fat is a component of the metabolic syndrome and plays an important role in CVD risk.15
At the beginning of treatment, we observed greater consumption of the fructose solution, which increased energy intake, with higher values at 30 and 60 days of life, but leveling out toward the end of treatment. Group F presented lower chow intake at 60 and 90 days of life.
Molecule for molecule, fructose provides the same energy as glucose, but it is not used directly by extrahepatic cells, since such cells do not express key enzymes that are required in the initial catabolic steps of producing energy from fructose.16 Thus, fructose's low energy efficiency may explain why the treatment did not result in body weight gain, as characterized by feed efficiency.
Food intake is modulated by limbic structures in the brain that establish feedback mechanisms with the hypothalamus, which are linked to the hedonic value of food intake.17 It is thus possible that consumption of fructose, which is pleasant tasting, could lead directly to changes in hunger and satiety mechanisms and in reward signals,18 and encourage energy intake irrespective of energy needs. In addition, fructose consumption can lead to resistance to leptin and insulin, two hormones that are involved in the hedonic response to food and other stimuli of the pleasure pathway.17
Various studies have investigated the effect of fructose in combinations with corn syrup or sucrose. We used a 20% pure fructose solution in water as the only drinking source in group F, while group C had water only, with no added sweetener. Other studies have used different forms of fructose and varying treatment periods, which makes comparison of results difficult.
No differences in serum glucose were observed between the groups, and while insulin was not assessed, it is possible that there was a compensatory increase in order to maintain euglycemia. The glycemic response to a high fructose intake suggests that it may trigger insulin resistance,3 but there have been conflicting results.
Studies in humans have demonstrated that consumption of fructose compared to glucose does not lead to systemic increases in glucose levels,19 which corroborates our findings in rats, although previous studies in both humans and animal models indicate that high consumption of fructose results in metabolic disturbances that together lead to insulin resistance.20
With regard to triglycerides, group F presented higher levels than group C. Hypertriglyceridemia is one of the most common disorders caused by a fructose-rich diet. High fructose consumption leads to a higher rate of de novo lipogenesis, a process that can increase the availability of fatty acids by directly affecting their synthesis.3,4
ConclusionFurther studies are needed to assess the effects of fructose, pure or in combinations of carbohydrates, as well as to compare it with other sugars, in order to clarify whether the effects described above are due to fructose itself or the increased energy intake resulting from its consumption, since data in the literature are conflicting. The present study identified the possible effects of fructose on abdominal adiposity and serum triglyceride levels. These two factors increase risk of CVD.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ramos VW, Batista LO, Albuquerque KT. Efeitos do consumo de frutose sobre ingestão alimentar, parâmetros bioquímicos e corporais em ratos Wistar. Rev Port Cardiol. 2017;36:937–941.