Constrictive pericarditis is a rare clinical entity that can pose diagnostic problems. The gold standard for diagnosis is cardiac catheterization with analysis of intracavitary pressure curves, which are high and, in end-diastole, equal in all chambers. The diastolic profile in both ventricles presents the classic dip-and-plateau pattern and the difference between the diastolic pressures of both ventricles should not exceed 3–5mmHg. Unfortunately, these traditional criteria are not always present and in fact the sensitivity and specificity of equalization of diastolic pressures are relatively low and of limited value in individual patients. This highlights the need to use new cardiac imaging techniques to resolve any doubts. The case described here is a good example.
A pericardite constritiva é uma entidade clínica rara que pode colocar problemas diagnósticos. O método padrão para a confirmação do seu diagnóstico é o cateterismo cardíaco, com análise das curvas de pressão intracavitárias, as quais estão elevadas e, em telediástole, são iguais em todas as cavidades. O seu perfil diastólico em ambos os ventrículos apresenta o clássico aspeto dip-plateau e a diferença entre as pressões telediastólicas ventriculares não deve exceder 3-5mmHg. Infelizmente estes critérios clássicos nem sempre se verificam e, na verdade, a sensibilidade e a especificidade da igualização das pressões telediastólicas dos ventrículos é relativamente baixa e de valor limitado no doente individualmente considerado. Esta realidade evidencia a necessidade de utilizar as novas técnicas de imagem cardíaca, que podem tornar claro o que é duvidoso. O caso clínico aqui descrito é um bom exemplo.
A 78-year-old white man was admitted for severe decompensated heart failure (NYHA class IV), predominantly right-sided, which had progressively worsened over the previous three months. He had a history of hypertension, type 2 diabetes and dyslipidemia and was a former smoker. He had been admitted to a different hospital eleven years previously with a large pericardial effusion, for which he underwent pericardiocentesis and 3000 ml of pericardial fluid compatible with exudate was drained, but the etiology was not determined, cytological, microbiological and bacteriological tests being negative. Chest computed tomography (CT) showed pleural and pericardial thickening, which was not investigated. The patient reported no history of tuberculosis and had a negative tuberculin test. He had remained asymptomatic until three months before the current hospitalization, when progressive and disabling global heart failure began.
On physical examination at admission to the cardiology department, the patient was hemodynamically stable (blood pressure 120/70mmHg), tachypneic (24cpm), not cyanotic (94% oxygen saturation in room air) but pale. His pulse was strong, irregular and symmetrical. Cardiac and pulmonary auscultation revealed a low intensity arrhythmia, an early third sound, a holosystolic mitral murmur radiating to the axilla and diminished breath sounds in the both lung bases, accompanied by pulmonary rales. There was marked jugular venous distension up to the mandibular angle, painless hepatomegaly (liver palpable 10cm below the right costal margin on the mid-clavicular line), positive hepatojugular reflux and lower limb edema up to the knee (+++/++++).
The electrocardiogram revealed atrial flutter with variable atrioventricular block and normal voltage. Laboratory tests showed marked elevation of erythrocyte sedimentation rate (ESR) (98mm) with no leukocytosis or neutrophilia, and low C-reactive protein (3mg/dl). Liver enzymes presented a pattern of passive congestion (alkaline phosphatase and gamma-glutamyl transferase higher than aminotransferase). NT-proBNP was normal (142pg/ml). The chest X-ray, in posteroanterior view, showed a normal cardiothoracic index and a small bilateral pleural effusion, and in profile view, a linear image located at the left ventricular (LV) lateral wall, of whitish appearance. Echocardiography performed at admission showed the entire pericardium to be thickened and hyperechogenic, no ventricular dilatation, moderately impaired LV global systolic function (ejection fraction [EF] 38% by Simpson's biplane method) and biatrial dilatation. There was mild mitral and tricuspid regurgitation, and tricuspid valve flow showed respiratory variation of around 30%. Mitral valve flow did not present significant respiratory variation; E-wave deceleration time was 120ms (mean of five complexes). Echocardiographic study also documented severe pulmonary hypertension (pulmonary artery systolic pressure [PASP] 70mmHg) and dilatation of the inferior vena cava (28mm), with reduced respiratory variation (<50%).
The main diagnostic hypothesis was constrictive pericarditis (CP). Concomitant coronary artery disease was also considered, since the patient had multiple cardiovascular risk factors and presented depressed left ventricular systolic function, as was pulmonary disease (in a former smoker with pleural thickening), which would explain the elevated PASP. Combined constrictive/restrictive cardiomyopathy could not be ruled out.
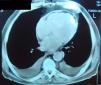
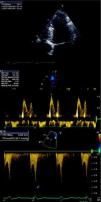
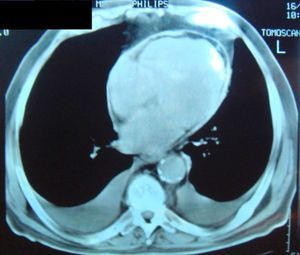
Complementary diagnostic exams included the latest echocardiographic modalities, notably assessment of LV strain and longitudinal early diastolic mitral annular velocity (E’). LV longitudinal strain was preserved, as was early diastolic mitral annular velocity (septal E’: 8.4cm/s; E/E’: 8.3) (Figure 1), with a respiratory variation of >25%. However, circumferential strain was reduced, a finding that supported a diagnosis of CP. As in the exam performed 11 years previously, chest CT showed diffuse pericardial thickening, but this time focal pericardial calcification was also observed. There were no significant parenchymal lung lesions (Figure 2). Cardiac magnetic resonance imaging (MRI) confirmed mild pericardial thickening in the basal and mid ventricular walls, reaching 3mm in the lateral LV wall (normal <2mm). Ventricular volumes and right ventricular EF were normal (LVEF 40%, moderately impaired), and there was mild mitral regurgitation. No delayed enhancement study was performed, as the patient did not wish to continue the exam.
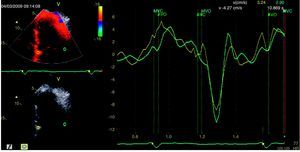
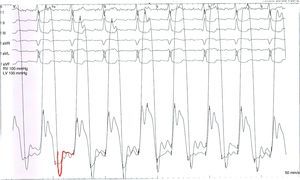
On the 6th day of hospital stay, hemodynamic assessment showed elevated LV end-diastolic pressure (22mmHg) and pulmonary artery wedge pressure (22mmHg) despite six days of diuretic therapy, a slightly diminished cardiac index (1.87l/min/m2), and persistent pulmonary hypertension (mean pulmonary artery pressure 38mmHg). Although the LV pressure curve presented a dip-and-plateau pattern, the right ventricular curve did not. Furthermore, end-diastolic pressures in the left chambers showed a difference of >5 mmHg compared to the right chambers (Figure 3), which did not support a diagnosis of CP. Coronary angiography excluded significant lesions and ventriculography showed impaired global systolic function (EF 45%) and generalized LV hypokinesia.
We arrived at a diagnosis of CP in the light of the clinical picture, laboratory tests (normal NT-proBNP) and noninvasive imaging studies (chest X-ray and CT and cardiac MRI). Some Doppler echocardiographic findings supported the diagnosis (restrictive pattern and tricuspid flow with respiratory variation >25%), while others did not (mitral flow without respiratory variation); the new echocardiographic modalities (2D strain and tissue Doppler of the mitral annulus) favored the principal hypothesis, unlike the hemodynamic study.
After clinical discussion, a diagnosis of CP prevailed and the patient was accordingly referred for surgical pericardial decortication, which was performed 19 days after admission.
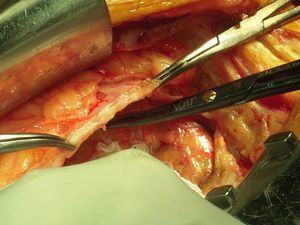
During the procedure, the thickened pericardial membrane was completely removed (Figure 4), and pleural and pulmonary biopsy specimens were taken. Anatomopathological study of the specimens showed marked fibrous thickening of the pericardium with focal congestion and nonspecific chronic inflammatory infiltrate, and several focal dystrophic calcifications; areas of pleural fibrous thickening with anthracotic pigment; and areas of emphysema and interstitial fibrosis, congestion and mild nonspecific chronic focal inflammatory infiltrate in pulmonary tissue.
Etiological studies performed during hospitalization (blood cultures, tuberculin testing, serum adenosine deaminase levels, and viral serology) were negative.
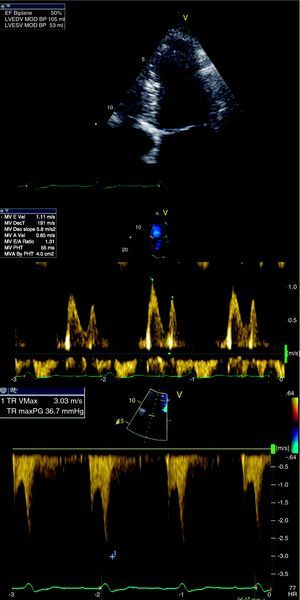
The postoperative period was uneventful and the patient was discharged from our hospital one month after admission, significantly improved, and was in NYHA functional class I after 29 months. The patient's echocardiogram (Figure 5), in sinus rhythm, showed improved global systolic function (EF 50% by Simpson's biplane method) and LV circumferential strain. E-wave deceleration time was 191ms (higher than initially) and the E/A ratio was 1.3, confirming improved diastolic function. There was no significant respiratory variation in valve flows and PASP was 42mmHg (right atrium/right ventricle gradient: 37mmHg), significantly lower than on initial assessment, probably due to improved LV systolic and diastolic function. The inferior vena cava presented normal caliber and respiratory variation. A month and a half after this last cardiovascular evaluation, the patient first presented rheumatoid arthritis.
DiscussionConstrictive pericarditis is a rare but serious consequence of chronic inflammation of the pericardium that results in a rigid fibrotic shell that constricts the cardiac chambers. The hemodynamic repercussions of this structural change include severe dysfunction of ventricular diastolic filling, which can be additionally aggravated by systolic dysfunction due to secondary myocardial fibrosis and atrophy.1 In the case presented, the patient had systolic and diastolic dysfunction due to the prolonged evolution of CP. There is usually a long interval between the initial pericardial lesion and the onset of constriction,1 which in our patient was 11 years.
A diagnosis of CP should always be considered in patients presenting with predominantly right heart failure symptoms, such as increased jugular venous pressure, pleural effusion, hepatomegaly, ascites and peripheral edema. Besides these systemic features, another characteristic of CP is the presence of an early third sound (pericardial knock), which results from a sudden cessation of ventricular filling due to pericardial constriction.2 In more severe cases, there is hypotension and circulatory collapse.
Conventional and Doppler echocardiography play an important role in diagnosing this entity, and in differential diagnosis with other causes of right ventricular (RV) failure,3 of which restrictive cardiomyopathy (RCM) is the most challenging to differentiate, since it has similar features on Doppler and hemodynamic study. The presence of LV systolic dysfunction in our patient, which is more common in RCM than in CP, made differential diagnosis essential. Dynamic respiratory variation in flow and enhanced ventricular interaction, which are found in CP but not RCM, are the most important pathophysiological processes for differential diagnosis.1 In CP, there is a dissociation of intrathoracic and intracardiac pressures, which results in a decrease in LV filling during inspiration. A rigid and noncompliant pericardium leads to enhanced ventricular interaction and hence increased RV filling during inspiration. Doppler and tissue Doppler echocardiography play a crucial role in analysis of these respiratory changes.1 When doubts remain, new echocardiographic modalities can help in diagnosis. Since in CP, the mechanical and elastic properties of the myocardium are relatively preserved longitudinally but attenuated circumferentially and, while rotation of the basal and mid segments is preserved, apical rotation is markedly reduced, it is assumed that longitudinal strain and early diastolic mitral annular velocity (E’) will be normal or increased (unlike in RCM), and that circumferential strain and the net twist angle (difference between apical and basal rotation) will be reduced (as in RCM). The net twist angle was not assessed in our patient.
Mitral E’ of >8cm/s differentiates CP from RCM with a sensitivity of 89% and specificity of 100%.4 Conventional, Doppler and 2D strain echocardiography were crucial for differential diagnosis between CP and RCM in our patient, and were also used to assess changes in cardiac morphology and function following pericardial decortication. Postoperative improvement in LVEF supports the hypothesis that systolic dysfunction was secondary to chronic inflammation and myocardial fibrosis arising from prolonged pericardial constriction, which is uncommon and thus makes our case somewhat unusual. Delayed enhancement cardiac MRI, which was not performed in our case due to the patient's refusal, would have detected any areas of intramyocardial fibrosis. Nevertheless, normal initial values for longitudinal strain and improved EF after surgery point to the absence of severe intramyocardial fibrosis.
Chest CT and cardiac MRI will detect a thickened pericardium in CP, but the information they provide is merely anatomical and does not necessarily reflect pathophysiological changes.5 In the case presented, these two exams supported our principal diagnostic hypothesis by documenting pericardial thickening with focal calcification. However, it should be remembered that 18% of patients with surgically proven CP present normal pericardial thickness and so the absence of thickening does not exclude CP.6
Measurement of NT-proBNP can also help in diagnosis, as patients with CP present normal (as in our case) or slightly elevated values, while those with RCM present very high levels.7 The fact that our patient recently developed rheumatoid arthritis may explain his elevated ESR, since alterations in laboratory analyses frequently precede symptoms in this type of disease.
With regard to hemodynamic findings, the patient's LV systolic and diastolic dysfunction explained his reduced cardiac index and elevated LV end-diastolic, pulmonary wedge, and pulmonary artery pressures, the latter probably exacerbated by concomitant lung disease (emphysema) documented on pulmonary biopsy. Various possibilities were considered to explain the absence of equalization of ventricular end-diastolic pressures in our patient, the most likely being volume depletion at the time of cardiac catheterization after six days of diuretic therapy. A bolus of additional fluid should be administered to confirm the presence of constriction, but this was not done as the patient had an episode of acute pulmonary edema in the context of hypertensive crisis the day before the hemodynamic study. Although cardiovascular catheterization, which can identify the typical hemodynamic behavior of CP, is considered the gold standard diagnostic method, in a recent study using high-fidelity, micromanometer-tipped catheters to differentiate between CP and RCM, the predictive power of conventional CP criteria was only 75%. The same study found that a new criterion – the systolic area index (which assesses the change in ventricular pressure area during inspiration and expiration and considers a ratio of more than 1.1 to be indicative of enhanced ventricular interaction) – had 97% sensitivity and 100% predictive accuracy for identifying patients with surgically proven CP.8 However, while the use of micromanometers may confer greater accuracy in assessing intracavitary pressures, they do not appear preferable to new cardiac imaging modalities, which are easier, quicker, noninvasive and reliable, and should be used in the diagnosis of doubtful cases of constrictive pericarditis.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Silvia D, et al. Pericardite constritiva – novos métodos no diagnóstico de uma velha doença: a propósito de um caso clínico. Rev Port Cardiol. 2012. doi:10.1016/j.repc.2012.07.004.