Cardiogenic shock is characterized by a decrease in myocardial contractility, and presents a high mortality rate. Inotropic and vasopressor agents have been recommended and used for several years in the treatment of patients in shock, but they remain controversial. Despite its beneficial effect on myocardial contractility, the side effects of inotropic therapy (arrhythmias and increased myocardial oxygen consumption) may be associated with increased mortality.
The pharmacodynamics of different inotropic agents suggest benefits in specific situations, but these differences have not been reflected in reduced mortality in most studies, making it difficult to formulate recommendations.
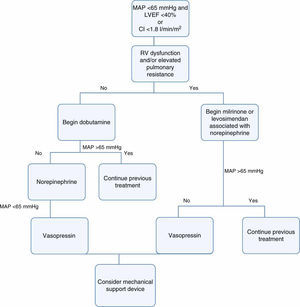
This review integrates data from different studies on the use of inotropes and vasopressors in patients with cardiogenic shock, proposing a therapeutic scheme for the pharmacological treatment of patients in cardiogenic shock according to the patient's hemodynamic profile.
O choque cardiogénico é caracterizado por uma diminuição da contratilidade miocárdica, apresentando uma mortalidade elevada. A instituição de terapêutica inotrópica e vasopressora é recomendada e utilizada há vários anos no tratamento de doentes em choque, mas continua a ser um tema controverso. Apesar do efeito benéfico na contratilidade miocárdica, os efeitos secundários dos inotrópicos (arritmias e aumento do consumo miocárdico de oxigénio) podem-se associar a um aumento da mortalidade.
A farmacodinâmica dos diferentes agentes inotrópicos sugere benefícios em determinadas situações, no entanto, estas diferenças não foram traduzidas em redução da mortalidade na maioria dos estudos, dificultando a criação de recomendações.
Esta revisão pretende integrar os dados dos diferentes estudos, com fármacos inotrópicos e vasopressores, em doentes com choque cardiogénico, sendo proposto um diagrama para o tratamento farmacológico de doentes em choque cardiogénico, de acordo com o perfil hemodinâmico do doente.
Cardiogenic shock (CS) is a state of impaired end-organ perfusion caused by a decrease in cardiac output despite adequate intravascular volume, and is usually associated with the following hemodynamic characteristics: systolic blood pressure of less than 90 mmHg for more than 30 min (in the absence of inotropic or vasopressor support), a reduction of cardiac index (<1.8 l/min/m2 without support and less than 2.2 l/min/m2 with support), and elevated left ventricular (LV) filling pressures (pulmonary capillary wedge pressure >18 mmHg).1,2
The most frequent cause of CS is acute myocardial infarction (AMI), accounting for almost half of cases; it complicates AMI in 5-15% of patients depending on the series.3 Since the SHOCK trial, primary percutaneous coronary intervention (PCI) has been the preferred treatment for AMI patients with CS.4 Advances in treatments, particularly revascularization, have reduced mortality from AMI and the incidence of CS, but CS is still associated with high mortality.3,5,6
Descriptions of the use of inotropes and vasopressors in CS go back to the 1950s,7,8 but there have been few clinical trials in these patients, and so the choice of which drugs to use remains unclear. We analyzed studies in the literature on inotropic and vasopressor agents, with the aim of determining the indications for the use of each drug. Table 1 shows the main drugs used in CS and their characteristics.
Drugs used in cardiogenic shock.
| Class | Mechanism of action | Half-life | Dose (infusion) | |
|---|---|---|---|---|
| Epinephrine | Catecholamine | α- and β-adrenergic blockade | 2 min | 0.01-1.0 μg/kg/min |
| Dobutamine | Catecholamine | β-adrenergic blockade | 2-3 min | 2-20 μg/kg/min |
| Dopamine | Catecholamine | α- and β-adrenergic and dopaminergic agonist | 2 min | 1-20 μg/kg/min |
| Milrinone | Phosphodiesterase inhibitor | Increases cAMP by inhibiting PDE3 | 2 h | 0.375-0.750 μg/kg/min |
| Levosimendan | Calcium sensitizer | Increases sensitivity of troponin C to intracellular Ca2+ | 1 h (metabolites up to 80 h) | 0.1-0.4 μg/kg/min |
| Norepinephrine | Catecholamine | α-adrenergic agonist | 2-2.5 min | 0.2-1 μg/kg/min |
| Vasopressin | Vasopressor | V1 and V2 vasopressin receptor agonist | 10-20 min | 0.6-6 UI/h |
cAMP: cyclic adenosine monophosphate; PDE: phosphodiesterase inhibitor.
Inotropic agents increase myocardial contractility, thereby increasing cardiac output. Many of these drugs also raise heart rate (HR) and thus myocardial oxygen consumption, which may be harmful in some patients. As well as their inotropic properties, they also have vasoconstrictive or vasodilatory effects.
Inotropes are currently used mainly to stabilize patients with acute heart failure (HF).
EpinephrineEpinephrine is a sympathomimetic hormone and drug that binds to α- and β-adrenergic receptors. It increases mean arterial pressure by increasing cardiac output and peripheral vascular tone,9 and is thus used to treat shock. Its effects in septic shock have been tested in clinical trials, in which it showed similar efficacy and impact on mortality to norepinephrine.10,11
In a trial of 30 patients with CS not due to acute coronary syndrome, Levy et al.12 compared epinephrine and combined norepinephrine-dobutamine. Efficacy, as measured by blood pressure (BP), oxygen consumption, improvement in renal function, and mortality were similar in the two groups. Patients treated with epinephrine showed a transient increase in lactate and blood glucose levels and higher HR; three patients in the epinephrine group presented tachyarrhythmias, but none in the norepinephrine-dobutamine group.
Morici et al.13 argue that epinephrine is important for hemodynamic stabilization of patients in CS and that it presents a better profile than other commonly used drugs, since for similar increases in BP, the balance between epinephrine's inotropic and vasoconstrictive effects means that the increase in HR is less marked than with dopamine, while vasoconstriction and hence afterload are less than with norepinephrine.
DobutamineDobutamine acts on the myocardium by stimulating β1-adrenergic receptors, increasing contractility, and on smooth muscle, acting on β2 receptors to cause vasodilation.14 It rapidly gained acceptance for the treatment of CS due to its ability to increase cardiac output and to reduce LV filling pressures.15,16
Francis et al.17 compared the hemodynamic effects of dopamine and dobutamine, reporting smaller increases in HR, fewer arrhythmias, less peripheral vasoconstriction and more consistent reductions in LV filling pressures with the latter for similar rises in cardiac output.
Richard et al.18 concluded that an infusion of dopamine and dobutamine achieved similar increases in cardiac output to dopamine alone but with lower oxygen consumption.
The efficacy of dobutamine has been compared to that of milrinone in hospitalized patients awaiting cardiac transplantation, with some authors reporting similar results19 and others favoring milrinone, in terms of mortality20 as well as less need for mechanical ventricular support.21
In a 2012 meta-analysis, Tacon et al.22 analyzed 14 trials with a total of 673 patients with severe HF, comparing dobutamine with placebo or standard care. Higher mortality was seen in patients treated with dobutamine, although without statistical significance.
DopamineDopamine is a natural precursor of norepinephrine and epinephrine. Its effects are dose-dependent: at low doses (1-2 μg/kg/min) it has a vasodilatory effect, binding to dopaminergic receptors, while at higher doses (5-10 μg/kg/min) it acts as a β1 receptor agonist and thus has inotropic effects. At even higher levels (>10 μg/kg/min) it stimulates α-adrenergic receptors, leading to vasoconstriction and an increase in BP.4,23
For a long time dopamine was thought to have a beneficial effect on renal function, but this was not observed in two trials24,25 and it is now not considered suitable as a treatment for renal dysfunction.
Although long used in CS, its popularity is now waning since a subgroup analysis of the SOAP II trial26 showed that patients with CS treated with dopamine had more arrhythmic events and higher mortality than those treated with norepinephrine. A possible explanation for this difference between norepinephrine and dopamine in shock is the weakened response to indirect agents such as dopamine due to neurotransmitter depletion in CS patients.27 However, a 2009 Portuguese study by Póvoa et al.28 demonstrated that dopamine was associated with lower mortality than norepinephrine in patients in septic shock.
MilrinoneMilrinone is a phosphodiesterase (PDE) inhibitor. By inhibiting PDE3, which degrades cyclic adenosine monophosphate (cAMP), it increases cAMP levels, promoting calcium uptake by cardiomyocytes and increasing myocardial contractility. In vascular smooth muscle, the reduced degradation of cAMP accelerates the removal of intracellular calcium, leading to relaxation and vasodilation.29 Milrinone is thus a positive inotrope and a peripheral vasodilator with lusitropic properties.
The fact that, unlike catecholaminergic agents, milrinone does not raise HR theoretically gives it the advantage of not increasing myocardial oxygen consumption.30
The first studies comparing milrinone with dobutamine31 indicated that the two drugs were equally effective in increasing cardiac output, but that reductions in left and right ventricular (RV) filling pressures and BP were more marked with milrinone.
Milrinone enables catecholamine doses to be reduced in catecholamine-dependent CS patients, facilitating weaning from prolonged catecholamine therapy. In a study by Siostrzonek et al.32 of 20 patients, milrinone improved weaning from catecholamines and enabled earlier discharge from the intensive care unit. However, vasopressor support needed to be increased in some patients.
The largest trial to date on milrinone, OPTIME-CHF,33 compared its efficacy to placebo in patients with acute exacerbation of chronic HF, although patients in shock were excluded. Milrinone was not shown to be superior in length of hospital stay or 60-day mortality and was associated with more cases of hypotension and atrial arrhythmias.
The ADHERE registry,34 the first results of which were published in 2005, showed that inotropes were associated with higher mortality than vasodilators, but only 2.5% of the patients had systolic BP <90 mmHg, so this finding cannot be extrapolated to patients in shock. The inotropes used were dobutamine and milrinone, mortality being higher in those under dobutamine.
In patients with severe HF needing prolonged use of inotropes, oral milrinone therapy on an outpatient basis was associated with an increase in mortality compared with placebo.35 However, milrinone appears to be more effective when association with β-blockers is tolerated.36 A more recent study compared the effectiveness of continuous intravenous infusion of milrinone with that of dobutamine; there was no significant difference in mortality in the two groups.37
The value of milrinone in individuals with reduced RV function and increased pulmonary vascular resistance was demonstrated by Pamboukian et al.38 and confirmed by Eicchorn et al.39 in a trial comparing milrinone with dobutamine, in which the former led to improvement in RV systolic function and reductions in pulmonary artery pressure.
LevosimendanLevosimendan is the most recently available inotrope for the treatment of acute HF. It increases the sensitivity of troponin C to intracellular calcium in the myocardium and thus has inotropic and lusitropic properties.40–42 It also acts on ATP-dependent potassium channels, leading to relaxation of vascular smooth muscle and hence coronary and peripheral vasodilation.34 Levosimendan's effects are dose-dependent and it has a demonstrated impact on the hemodynamic profile of HF patients43; the effects of its active metabolites can last for up to five days after discontinuation of the drug.44
Some studies45–50 have shown that in patients with severe HF levosimendan does not affect mortality compared to placebo, and the results are contradictory when it is compared to other inotropes. In the REVIVE II trial,45 the levosimendan group had rapid symptomatic relief and a reduction in brain natriuretic peptide levels when levosimendan was added to standard treatment, but with a numerically higher risk of death.
In the LIDO study,46 levosimendan was compared to dobutamine in patients with low-output HF and need for inotropic support. At 180 days, patients taking levosimendan presented lower mortality and more days to rehospitalization. The LEAF trial47 assessed the efficacy of levosimendan compared to placebo in patients undergoing primary PCI following AMI complicated by HF and revealed improvements in myocardial contractility but no reduction in mortality. Nine patients in this trial had CS but there were no significant differences between them and the other patients.
A Portuguese study on levosimendan, the PORTLAND study,48 which included 129 patients with acute HF and LV dysfunction but excluded those with CS, found that the drug was safe, improved symptoms and reduced readmissions at six-month follow-up. However, this study did not compare levosimendan with other therapies, but only with national data on HF admissions.
Samimi-Fard et al.49 compared outcomes at 12 months in 22 AMI patients with CS after primary PCI treated with levosimendan or dobutamine, and found no significant differences between the groups. Fuhrmann et al.,50 comparing levosimendan and enoximone in 32 CS patients, reported lower mortality at 30 days in the levosimendan group.
In addition to its effects on LV function, levosimendan has also been shown in a small study on 25 patients51 to improve RV function and to reduce pulmonary vascular resistance. It may therefore be useful in AMI patients with CS and RV dysfunction.
VasopressorsVasopressors are the first-line treatment for patients in shock with low systemic vascular resistance and unresponsive to fluid therapy. In CS, a proinflammatory state may persist that leads to vasoplegia,52 requiring the use of vasopressors to maintain BP. Vasoconstrictive inotropes such as dopamine and epinephrine may be used for this purpose, but the two vasoconstrictors that are most used nowadays are norepinephrine and vasopressin (Table 1). The drug with the greatest impact on mortality in patients with shock is norepinephrine.26
NorepinephrineThe catecholamine norepinephrine is an α-adrenergic agonist that is used as a vasopressor to increase BP in patients with shock. The first report of a patient with CS following AMI treated with norepinephrine appeared in 1953.7 Although the patient died (from CS complicated by sepsis), hemodynamic improvement had been seen after norepinephrine infusion.
The adverse effects associated with norepinephrine use, such as reduced renal and splanchnic blood flow, especially in patients needing volume expansion, are well known.53
The European Society of Cardiology (ESC) guidelines for the management of ST-segment elevation AMI54 are unclear on treatment for patients in CS, stating that when blood pressure is low, norepinephrine should be the first choice, but gives dopamine a class IIa recommendation, level of evidence C, and norepinephrine class IIb/B.
As stated above, in the SOAP II trial,26 norepinephrine was associated with lower mortality than dopamine in CS patients, and is thus considered the first-line treatment, possibly together with an inotrope.
VasopressinVasopressin is a hormone that binds to its own receptors. Binding to V1 receptors causes vasoconstriction due to contraction of vascular smooth muscle,23,55 while V2 stimulation increases renal free water reabsorption.55 In association with norepinephrine, it has been shown to be effective and safe for treating patients in septic shock,23 enabling dosages of other vasopressors to be reduced.55
In situations of cardiopulmonary arrest, vasopressin has similar efficacy to epinephrine for resuscitating patients in ventricular fibrillation or pulseless electrical activity, and is more effective in the presence of asystole.56
A small retrospective study57 of CS patients showed that in those under dopamine therapy, adding vasopressin was not inferior to norepinephrine as assessed by increases in mean BP and other hemodynamic parameters, although no benefit was seen in terms of mortality.
A good response to vasopressin was also observed in hypotensive patients after LV assist device placement, reducing their need for norepinephrine.58
However, to date there have been no randomized clinical trials on vasopressin in CS patients.
DiscussionThe range of studies on the use of these drugs in CS (Table 2) clearly demonstrates the many uncertainties surrounding the subject.
Studies on inotropes and vasopressors in cardiogenic shock.
| Study and year | Objectives | Study type | No. of patients | Population | Endpoints | Results | |
|---|---|---|---|---|---|---|---|
| Inclusion criteria | Exclusion criteria | ||||||
| Levy et al., 201112 | To compare hemodynamic effects, lactate metabolism and impact on systemic perfusion of epinephrine and combined dobutamine-norepinephrine | Randomized | 30 | CI <2.2 l/min/m2 MAP <60 mmHg Signs of systemic hypoperfusion No hypovolemia | ACS | HR MAP pCO2 in gastric mucosa Lactate level | Epinephrine and norepinephrine-dobutamine had similar hemodynamic effects Epinephrine associated with transient increase in lactate level and HR, and inadequate gastric mucosa perfusion |
| Myburgh et al., 200810 | To determine differences between epinephrine and norepinephrine in achieving MAP goal in ICU patients | Randomized | 280 | ICU patients requiring norepinephrine or epinephrine | Post cardiac arrest Anaphylactic reaction to either drug Pheochromocytoma Low epinephrine levels Treatment with MAO inhibitors | Time to achieve MAP goals 28- and 90-day mortality | No differences in any endpoints |
| Annane et al., 200711 | Efficacy and safety of norepinephrine plus dobutamine vs. epinephrine in septic shock | Randomized | 330 | Patients diagnosed with septic shock | Pregnancy Obstructive cardiomyopathy ACS Other types of shock | 28-day mortality | Similar mortality in both groups |
| Francis et al., 198217 | To compare hemodynamic effects of dopamine and dobutamine in patients with CS | Randomized | 13 | Patients diagnosed with CS | Other types of shock | HR Arrhythmias Peripheral perfusion LV filling pressures CO | Dobutamine increased stroke index and CI more than dopamine. |
| Richard et al., 198318 | To assess the efficacy of combined dobutamine and dopamine in CS | Randomized | 8 | Patients in CS under invasive ventilation | NA | MAP PCWP CI HR | The dopamine-dobutamine combination increased MAP and maintained PCWP within normal limits |
| Aranda et al., 200319 | To compare clinical outcomes and costs associated with the use of dobutamine or milrinone in hospitalized patients awaiting cardiac transplantation | Randomized | 36 | Inotrope-dependent patients awaiting cardiac transplantation | Intolerance to dobutamine or milrinone Hemodynamic instability requiring mechanical support device | Hemodynamic profile Ventricular arrhythmias Need for additional vasodilators or inotropes | No clinical differences between the two drugs |
| Mehra et al., 199721 | To assess the safety of IV milrinone for >72 h and its utility as a bridge to cardiac transplantation in advanced HF | Observational | 71 | Dependence on inotropic therapy Hemodynamically stable for ≥72 h IV milrinone for >72 h | NA | Need for mechanical circulatory support Cardiac adverse effects | Milrinone was safe for periods of >72 h, and may be associated with less need for mechanical ventricular support |
| SOAP II De Backer et al., 201026 | To determine whether norepinephrine or dopamine as first-line vasopressor therapy reduces mortality in patients with shock | Randomized | 1679 | MAP <70 mmHg after at least 1000 ml of crystalloids or 500 ml of colloids and signs of tissue hypoperfusion | Age <18 years Already received vasopressor agent for >4 h Serious arrhythmia such as rapid atrial fibrillation or VT Brain death | Mortality at 28 days, 6 and 12 months Adverse events | Similar mortality with the two drugs More arrhythmias in the dopamine group Subgroup analysis showed higher mortality with dopamine in CS than norepinephrine |
| SACiUCI Póvoa et al., 200928 | To assess the impact of choice of vasopressor support on mortality in septic shock | Observational | 458 | All adult patients admitted for septic shock | NA | In-hospital and 28-day mortality | Higher in-hospital and 28-day mortality with norepinephrine than with dopamine |
| Colucci et al., 198631 | To assess the hemodynamic effects of milrinone and dobutamine in advanced HF | Prospective Non-randomized | 15 | Patients in NYHA class III-IV | NA | CI LV and RV filling pressures Systemic vascular resistance | Similar increases in CI Milrinone showed greater reduction in LV and RV filling pressures and systemic vascular resistance |
| OPTIME-CHF Cuffe et al., 200233 | To assess the impact of milrinone vs. placebo in addition to standard therapy in patients hospitalized with an exacerbation of chronic HF | Randomized | 949 | Patients hospitalized for HF in NYHA class II-IV LVEF <40% | Need for IV inotropes ACS AF with poor ventricular rate control VT or VF | No. of days hospitalized within 60 days of randomization Adverse events | No difference in days hospitalized More adverse events with milrinone |
| ADHERE Adams et al., 200534 | To compare mortality in patients hospitalized with acute HF medicated with one of four vasoactive agents: nitroglycerin, nesiritide, milrinone or dobutamine | Registry | 65180 | Patients hospitalized with acute decompensated HF | HF is not the principal focus of diagnosis | In-hospital mortality Length of hospital stay | Greater mortality and length of hospital stay in patients treated with inotropes than in those treated with vasodilators (mortality: milrinone 12.3%, dobutamine 13.9%, nitroglycerin 4.7%, nesiritide 7.1%) |
| PROMISE Packer et al., 199135 | To determine the effect of oral milrinone on the mortality of patients with severe chronic HF who remain symptomatic despite conventional therapy | Randomized | 1008 | Patients in NYHA class III-IV, with LVEF <35% and receiving treatment with digoxin, diuretics, and ACE inhibitors | Obstructive valvular disease, active myocarditis, hypertrophic or amyloid cardiomyopathy, uncorrected thyroid disease | All-cause mortality In-hospital cardiovascular mortality | Increased mortality in patients treated with milrinone |
| Gorodeski et al., 200937 | To compare the effect on mortality of milrinone vs. dobutamine in patients with HF on continuous inotropes | Case-control | 112 | Inotrope-dependent patients with HF | NA | Survival | No mortality differences between dobutamine and milrinone |
| Pamboukian et al., 199938 | To determine the effects of milrinone on pulmonary vascular resistance | Observational Retrospective | 19 | Patients with pulmonary hypertension being assessed for cardiac transplantation | NA | Pulmonary vascular resistance CO Pulmonary artery pressure PCWP | Milrinone reduced pulmonary vascular resistance, pulmonary artery pressure and PCWP and increased CO |
| REVIVE II Packer et al., 201345 | To evaluate the efficacy and safety of levosimendan in patients with acute HF | Randomized | 700 | Patients admitted for acute HF LVEF <35% | Orotracheal intubation SBP ≤90 mmHg or HR >120 bpm Angina within 6 h or cardioversion within 4 h Valvular obstruction Stroke or TIA within 3 months Severe hepatic impairment Severe renal insufficiency (serum creatinine >5 mg/dl) Severe COPD Anemia (Hb <10 g/dl) Active infection Serum potassium concentration <3.5 or >5.4 mmol/l History of torsades de pointes | Death Symptoms Worsening HF Plasma BNP | Similar 14-day mortality Levosimendan group: - earlier hospital discharge - greater reduction in BNP levels at 5 days - similar BNP levels at 31 days |
| LIDO Follath et al., 200246 | To compare the effects of levosimendan and dobutamine on hemodynamic performance and clinical outcome in patients with low-output HF | Randomized | 203 | Hospitalization for low-output HF and need for inotropes Worsening of chronic HF despite optimal therapy Severe HF following cardiac surgery Acute HF with LVEF <35% CI <2.5 l/min/m2 PCWP >15 mmHg | Age <21 years Women of child-bearing age Hypertrophic or restrictive cardiomyopathy Stenotic valve disease Chest pain at time of randomization VT/VF in previous 2 weeks HR >120 bpm SBP <85 mmHg Severe renal failure Hepatic failure Cardiac tamponade Septic shock ARDS | Hemodynamic improvement at 24 h: increase of 30% or more in CO and a decrease of 25% or more in PCWP Mortality No. of days to readmission | Greater hemodynamic improvement with levosimendan than with dobutamine Lower mortality at 180 days with levosimendan (26% vs. 38%) |
| LEAF Husebye et al., 201247 | To evaluate the efficacy and safety of levosimendan in patients with primary PCI-treated STEMI complicated by symptomatic HF | Prospective Non-randomized | 61 | STEMI patients following primary PCI with: - revascularization by PCI - alterations in at least 3 LV segments HF with one of the following: - acute pulmonary edema - need for CPAP or invasive ventilation or IV diuretics due to congestion - persistent oliguria - shock with SBP <90 mmHg and systemic hypoperfusion | Age <20 years HR >120 bpm Septic shock ARDS Creatinine >450 μmol/l Severe hepatic failure Significant LVOT obstruction Allergy to one of the drugs Anemia Pregnancy | Change in wall motion score index Changes in NT-proBNP levels Infarct size at 42 days Time to MACE (death, non-fatal AMI, revascularization, rehospitalization for HF) | Levosimendan improves wall motion score index following AMI Similar MACE in the two groups |
| PORTLAND Silva-Cardoso et al., 200948 | To evaluate the clinical effectiveness and safety of levosimendan in the treatment of acute systolic HF in daily practice | Prospective Non-randomized | 129 | Patients in NYHA class III-IV, LVEF <40%, decompensated HF requiring inotropes | Shock Uncontrolled tachyarrhythmia Resting or post-infarction angina History of torsades de pointes Mechanical obstruction of LV filling or ejection Severe renal failure (serum creatinine >3 mg/dl) Severe hepatic failure Anemia (Hb <9 g/dl) Pregnancy Hypersensitivity to levosimendan | Proportion of patients in whom levosimendan is clinically effective and safe at 24 h Proportion of patients in whom both clinical effectiveness and safety were observed at 5 days | Levosimendan was effective and safe, with apparent symptomatic improvement Reduction in number of days of hospitalization at 6 months with levosimendan |
| Samimi-Fard et al., 200749 | To assess the effect on long-term survival of levosimendan compared to dobutamine in patients with STEMI revascularized by PCI who subsequently developed CS | Randomized | 22 | STEMI patients treated by PCI who developed CS | RV dysfunction VT Significant mitral regurgitation | 12-month mortality | Similar 12-month mortality in the two groups |
| Fuhrmann et al., 200850 | To investigate the effects of levosimendan compared with enoximone in refractory CS complicating AMI | Randomized | 32 | CS refractory to revascularization, IAAB, fluid therapy and inotropes CI <2.5 l/min/m2 PCWP ≥8 mmHg Signs of hypoperfusion | Mechanical complications of AMI Stenotic valve disease VT Major bleeding Severe hepatic failure Sepsis CS for >24 h | 30-day mortality | Lower mortality with levosimendan than with enoximone (31.3% vs. 62.5%) |
| Russ et al., 200951 | To assess the hemodynamic effects of levosimendan on LV and RV function in patients with CS following AMI | Observational | 25 | AMI patients with CS | NA | CI and RV cardiac power index Pulmonary vascular resistance | Levosimendan improves hemodynamic parameters of RV and LV performance |
| Jolly et al., 200557 | To examine the effects of vasopressin on CI and urine output in patients with CS after AMI | Retrospective | 30 | Patients in CS within 5 days of an AMI, under dopamine therapy that required the addition of norepinephrine or vasopressin and under pulmonary artery catheter monitoring | NA | MAP CI PCWP | Vasopressin was associated with increased MAP and had no adverse effect on CI or PCWP |
ACS: acute coronary syndrome; ACE: angiotensin-converting enzyme; AF: atrial fibrillation; AMI: acute myocardial infarction; ARDS: adult respiratory distress syndrome; BNP: brain natriuretic peptide; CPAP: continuous positive airway pressure; CI: cardiac index; CO: cardiac output; COPD: chronic obstructive pulmonary disease; CS: cardiogenic shock; Hb: hemoglobin; HF: heart failure; HR: heart rate; IAAB: intra-aortic balloon pump; ICU: intensive care unit; IV: intravenous; LV: left ventricular; LVEF: left ventricular ejection fraction; LVOT: left ventricular outflow tract; MACE: major adverse cardiac events; MAO: monoamine oxidase; MAP: mean arterial pressure; NA: not available; pCO2: partial pressure of carbon dioxide; NYHA: New York Heart Association; PCWP: pulmonary capillary wedge pressure; PCI: percutaneous coronary intervention; RV: right ventricular; SBP: systolic blood pressure; STEMI: ST-elevation myocardial infarction; TIA: transient ischemic attack; VF: ventricular fibrillation; VT: ventricular tachycardia.
On analysis of these results, no inotropic agent is reported as superior to placebo, although some have beneficial effects compared to other drugs. This may be partly because in placebo-controlled studies, what actually happened was that the drug was added to the center's standard treatment, and the additive effect of inotropes was not shown to reduce mortality in advanced HF.
Nevertheless, in the absence of alternatives, inotropes and vasopressors continue to be essential in the management of patients in CS,59 in order to prevent tissue hypoperfusion and the resulting organ dysfunction by maintaining a mean arterial pressure of 65-70 mmHg.60
The ESC guidelines on HF61 recommend the use of an inotrope such as dobutamine for hypotensive (‘shocked’) patients (class IIa recommendation) and a vasopressor (e.g. norepinephrine or dopamine) in patients under inotrope therapy to increase blood pressure and improve vital organ perfusion (class IIb). In the American College of Cardiology Foundation/American Heart Association (ACC/AHA) HF guidelines,62 inotropic support is recommended (class I) for patients with CS, while the use of vasopressors is mentioned in the text without class of recommendation; in neither case are specific drugs recommended.
At present, the use of drugs in CS varies according to the experience of physicians and the conditions in each center, as demonstrated by Pei et al.,63 who suggest that medical training in vasoactive agent use for shock management needs to be improved and treatment should be standardized.
In view of all of the above and in light of current knowledge, the authors believe that inotropes and vasopressors should be used in CS at minimum doses and for only as long as absolutely necessary, in accordance with the therapeutic scheme outlined in Figure 1.
As suggested in the ESC guidelines54 and following publication of the results of the SOAP II trial,26 norepinephrine should be the first-line drug for CS patients with hypotension and vasoplegia. Adding vasopressin should be considered in those needing high doses of norepinephrine and in those with an unstable heart rhythm, in whom raising the norepinephrine dose would be unsafe.
Inotropes should be reserved for short-term treatment in cases of low cardiac output. Dobutamine should be used in patients with isolated LV dysfunction, while those with elevated pulmonary resistance and RV dysfunction appear to benefit from a phosphodiesterase inhibitor such as milrinone or levosimendan. These two drugs are also the best option in patients under beta-blocker therapy, since their action is independent of β-adrenergic receptors.
Epinephrine can also be used in CS and is equally safe, and so may be added to the above drugs if they do not achieve a rise in BP.
Dopamine appears to have the most adverse effects and is of little apparent benefit, and so its role in shocked patients is increasingly limited; the ACC/AHA guidelines on ST-elevation myocardial infarction64 do not suggest any specific inotrope or vasopressor, but do emphasize that dopamine should be avoided.
The ideal inotrope would increase cardiac output and reduce ventricular filling pressures, have no adverse effects and reduce mortality. The search continues for such a drug for the treatment of CS.65 Omecamtiv mecarbil is a promising new drug for stable HF that exerts inotropic effects by activating cardiac myosin.29 Gene therapy is another area in which innovatory treatments for HF are being developed, with promising results in phase II trials.66 Further results of these new approaches are awaited.
In the IABP-SHOCK II trial,67 mortality in patients with CS complicating AMI was around 40% despite inotropic and vasopressor therapy, even with the benefit of intra-aortic balloon counterpulsation. Mortality in CS patients occurs mainly in the first three days,6,67 and so as well as medical therapy, mechanical circulatory support devices should be considered as soon as possible. The results of studies on such devices68 are promising in hemodynamic terms and they are recommended for patients in persistent shock after inotropic and vasopressor therapy. Even so, mortality in these patients remains high, implying that this approach does not always deliver the intended benefits.69,70 Trials are under way to determine which are the best devices for particular situations and when they should be implanted.
In 2009 data from the Portuguese national acute coronary syndromes registry were published,71 including 22482 patients between 2002 and 2008. In this registry, 7% of patients were medicated with catecholamines, 50% of them for CS. Although precise data on the use of mechanical support devices in Portugal are lacking, the number of patients with access to this therapy is small, and there would undoubtedly be benefits from the establishment of referral networks for CS.
ConclusionsThe only treatment shown to reduce mortality in CS is emergent revascularization.
The choice of drugs to treat CS is still controversial and more randomized trials in this area are needed.
Patients in CS refractory to medical therapy have a poor prognosis and some may benefit from mechanical circulatory support.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Amado J, Gago P, Santos W, Mimoso J, de Jesus I. Choque cardiogénico – fármacos inotrópicos e vasopressores. Rev Port Cardiol. 2016;35:681–695.