Anomalous pulmonary venous connection is an uncommon congenital anomaly in which all (total form) or some (partial form) pulmonary veins drain into a systemic vein or into the right atrium rather than into the left atrium.
The authors present one case of total anomalous pulmonary venous connection and two cases of partial anomalous pulmonary venous connection, one of supracardiac drainage into the brachiocephalic vein, and the other of infracardiac anomalous venous drainage (scimitar syndrome).
Through the presentation of these cases, this article aims to review the main pulmonary venous developmental defects, highlighting the role of imaging techniques in the assessment of these anomalies.
A conexão anómala das veias pulmonares é uma anomalia congénita rara na qual todas as veias pulmonares (forma total) ou algumas (forma parcial) drenam numa veia sistémica ou na aurícula direita, em vez da aurícula esquerda.
Os autores apresentam um caso de conexão anómala total das veias pulmonares e dois casos de retorno anómalo parcial das veias pulmonares, uma drenagem supracardíaca ao nivel da veia braquiocefálica e uma infracardíaca (síndrome da cimitarra).
Através da apresentação destes casos, este artigo pretende fazer uma revisão dos principais defeitos do desenvolvimento venoso pulmonar e realçar a importância das técnicas de imagem na avaliação destas anomalias.
Anomalous pulmonary venous connections are a specific group of congenital heart defects caused by the abnormal drainage of a part or the entire lung to a systemic vein or the right atrium. The estimated incidence is 2/100000 births.1 Most frequently only a single pulmonary vein is anomalous. However, more than one vein can have abnormal drainage, and rarely all the pulmonary venous vessels can connect to the right side of the heart, a condition known as total anomalous pulmonary venous connection (TAPVC).
We report the cases of three children with anomalous pulmonary venous connections: two of partial anomalous pulmonary venous connection (PAPVC) with an indolent course, and a neonate with TAPVC requiring urgent surgical intervention.
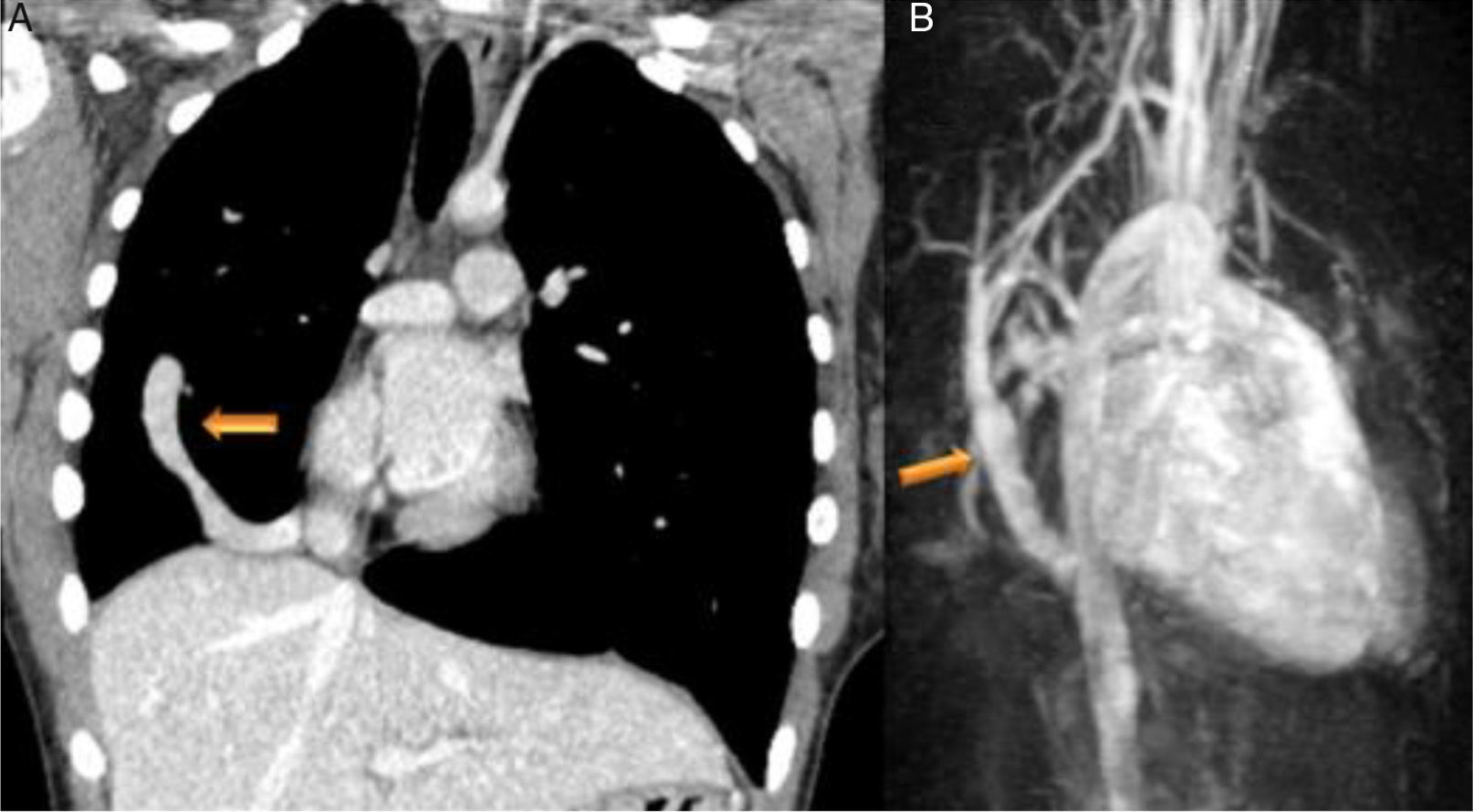
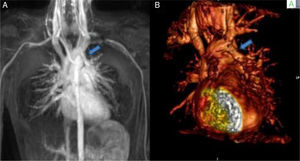
Case reportsCase 1 (partial anomalous venous return with infracardiac drainage)We describe the case of a 14-year-old girl assessed for an incidental finding on chest X-ray (pulmonary asymmetry). The chest X-ray revealed right pulmonary hypoplasia as well as an anomalous pulmonary vein descending below the diaphragm creating a curved shape on the right side, the scimitar sign. Echocardiography showed dextrocardia with apex on the left, at least two pulmonary veins draining into the left atrium, intact atrial and ventricular septa, no dilation of the cardiac chambers, preserved global biventricular systolic function and no signs of pulmonary hypertension. Computed tomography (CT) confirmed and better characterized these imaging findings (Figure 1A). Magnetic resonance imaging (MRI) (Figure 1B), in addition to angiographic evaluation, was also important for excluding associated congenital heart disease, and for assessment of right ventricular (RV) systolic function and volume, as well as left-to-right shunting (Qp:Qs 1:2). Given that the patient was asymptomatic and there was no evidence of cardiac functional impairment, a conservative strategy was adopted.
(A) Coronal-reformatted contrast-enhanced computed tomography showing the scimitar vein draining into the inferior vena cava (arrow) as well as right pulmonary hypoplasia with left lung expansion; (B) sagittal four-dimensional magnetic resonance imaging angiographic image also demonstrating the scimitar vein (orange arrow).
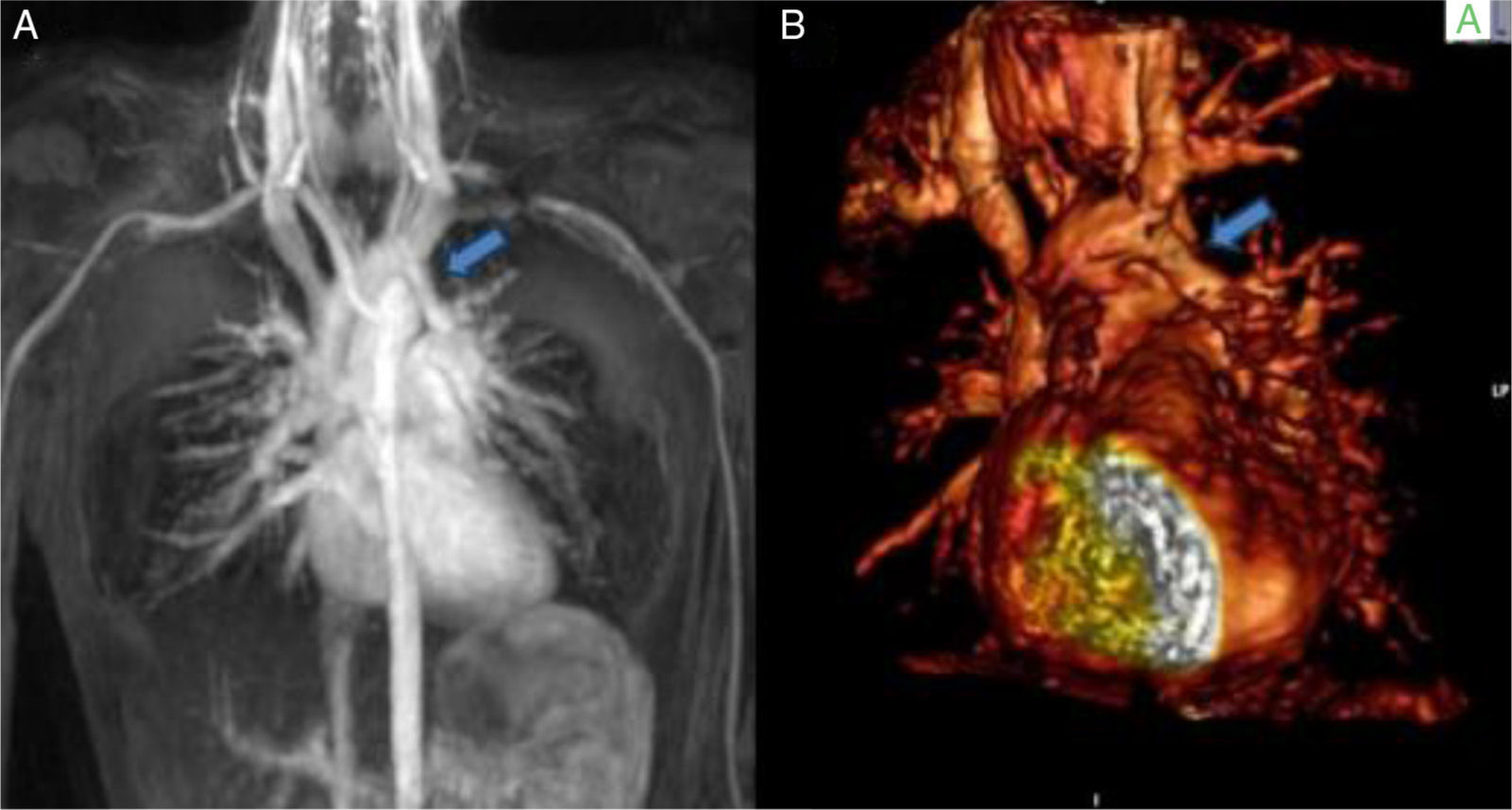
A nine-year-old girl was referred to the pediatric cardiology department for a cardiac murmur and Turner syndrome. She was asymptomatic and presented an ejection systolic murmur at the left sternal border. The electrocardiogram (ECG) showed right bundle branch block and the echocardiogram showed no intracardiac shunts, right ventricular dilatation with abnormal motion of the ventricular septum, and normal biventricular function. Cardiac catheterization revealed a normal heart with Qp/Qs 1:1. She continued to be followed in the pediatric cardiology clinic. At the age of 13 years she still presented right ventricular dilatation with an intact interatrial septum. MRI (Figure 2A and B) showed a partial anomalous pulmonary venous connection to the brachiocephalic vein, intact interatrial septum, right ventricular dilatation and Qp/Qs 1.6:1. She underwent cardiac surgery with a good outcome. This case highlights the potential role of MRI in these anomalies and is a reminder that although it is still the gold standard, cardiac catheterization when performed incorrectly may give inaccurate information. One of the key points is to take venous blood samples at various levels in order to obtain precise results.
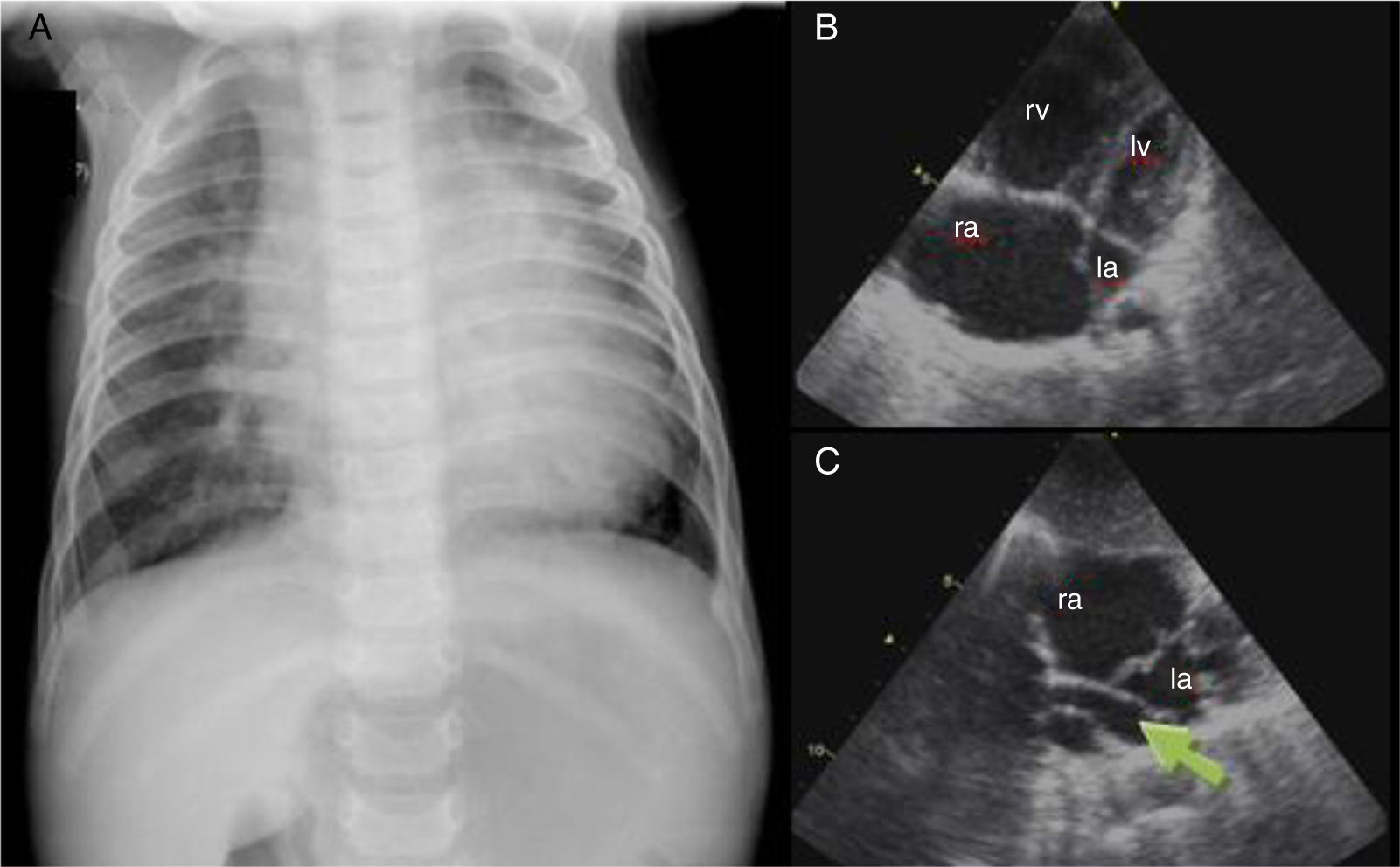
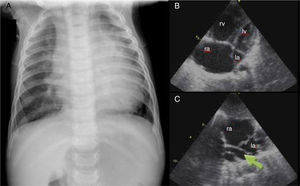
Case report 3 (total anomalous pulmonary venous connection)A six-month-old male infant was referred to our hospital for respiratory distress and failure to thrive. Physical examination showed transcutaneous oxygen saturation 90%, respiratory rate 50 cpm, and subcostal and inferior intercostal retractions. Symmetrical and wide peripheral pulses were observed, a faint systolic murmur was audible over the left sternal border, and the liver was palpable 2 cm below the right costal margin. The chest X-ray (Figure 3A) showed cardiomegaly and moderate pulmonary venous congestion. Echocardiography (Figure 3B and C) showed a dilated right atrium and ventricle, a small left atrium and a small ostium secundum type atrial septal defect (ASD). No pulmonary venous connection to the left atrium could be identified, and there was evidence of an abnormal pulmonary venous confluence behind the left atrium. A diagnosis of TAPVC was made. The patient underwent surgery in order to redirect pulmonary vein flow to the left atrium and to close the ASD. Since then, his growth and development have been appropriate for his age.
(A) Chest X-ray showing cardiomegaly and moderate pulmonary venous congestion; (B) dilatation of the right chambers; (C) total anomalous pulmonary venous connection, with no communication between the pulmonary veins and left atrium and a common vertical vein (arrow). la: left atrium; lv: left ventricle; ra: right atrium; rv: right ventricle.
The development of the pulmonary veins starts early in embryonic life and involves several complicated processes. Pulmonary venous developmental anomalies occur if any of these processes fails to progress properly. Thus, abnormal venous pulmonary drainage may be partial (PAPVC) in cases where only part of the pulmonary venous anatomy is abnormal, or it can involve all the pulmonary veins (TAPVC), resulting in complete drainage of the pulmonary venous return into the systemic venous circulation.
TAPVC, as in case report 3, accounts for approximately 1-5% of congenital cardiovascular anomalies.2 This condition is a cause of neonatal cyanosis and may rapidly result in death when blood is not shunted from the pulmonary to the systemic circulation. This shunting typically occurs through an ASD or patent foramen ovale or, less commonly, a patent ductus arteriosus.3
PAPVC is also a relatively uncommon congenital anomaly, found in only 0.5–0.7% of the general population.4 Anomalies in veins from the right lung are twice as common as from the left lung. The most common form is one in which a right superior pulmonary vein connects to the right atrium or the superior vena cava. This form is almost always associated with a sinus venosus type ASD. PAPVC with an intact atrial septum, as in case report 2, is a very rare finding.
Anomalous pulmonary venous connections are classified on the basis of the location of pulmonary venous drainage as one of four types: supracardiac, cardiac, infracardiac, or mixed.
In case report 1, we describe an infracardiac pulmonary drainage known as scimitar syndrome, in which part of the right lung is drained by a pulmonary vein connecting to the inferior vena cava. This anomaly is usually found in combination with hypoplasia of the right lung, pulmonary hypertension and other cardiac defects. Overall, 19-31% of patients with scimitar syndrome have associated cardiac anomalies.5 Its name refers to the tubular opacity typically following the right cardiac border, which resembles the curved Turkish sword known as a scimitar. This syndrome can present early in the neonatal period or later in life with a wide clinical spectrum. When diagnosis is established beyond the neonatal period, symptoms are usually milder or even absent, depending on the degree of lung hypoplasia.
Patients with PAPVC are typically acyanotic and most commonly only mildly symptomatic or asymptomatic. Some authors have suggested that PAPVC becomes clinically significant only when 50% or more of the pulmonary blood flow returns anomalously.6
Imaging techniquesImaging techniques, especially CT and MRI, have a pivotal role in the accurate characterization of these abnormalities, predicting outcomes and establishing appropriate preoperative planning. Echocardiography is the initial imaging technique of choice for congenital heart disease, but it has several limitations in the detection and assessment of these anomalies. Besides, pulmonary angiography by right heart catheterization may not reveal the anatomical details of small accessory and anomalous vessels. CT offers the possibility of noninvasive and rapid acquisition with high resolution. Both axial and three-dimensional reconstructed images depict anomalous pulmonary venous structures clearly, with statistically similar detection rates that approach 100%.3 Cardiac gating is not required for the assessment of pulmonary venous structures, although it may prove useful if the patient is being assessed specifically for cor triatriatum or central pulmonary vein hypoplasia or stenosis. The primary disadvantage of CT is that it requires the use of ionizing radiation, which is a major concern particularly in young patients. The radiation exposure from a single diagnostic procedure is usually harmless. However, because of the increased lifetime risk per unit dose for children, radiation can lead to a small, but non-negligible, increase in risk of cancer. The use of ECG-gated dose modulation may limit exposure during the less informative parts of the cardiac cycle.
MRI has the advantages of not using ionizing radiation and the ability to acquire multiple imaging phases using a single intravenous bolus of gadolinium contrast, and is capable of depicting associated cardiac defects. Moreover, MRI can quantify cardiac volumes and cardiac function, being particularly useful for the assessment of RV function, as well as valve dynamics. A variety of MRI techniques can be used to evaluate the pulmonary venous system. High-resolution double inversion-recovery fast (or turbo) spin echo (or black blood) images are useful for assessing anatomy. Gradient-recalled echo and two-dimensional balanced steady-state free precession provide important information about cardiac chamber and valvular function.3 Gadolinium-enhanced MRI angiography is an extremely useful technique since it is independent of ECG gating and allows rapid dynamic imaging of thoracic vascular structures. In addition, phase-contrast MRI has been validated as an accurate method for noninvasive quantification of intracardiac shunting.7 Disadvantages of MRI include the amount of time required for image acquisition, the frequent need for patient sedation, and its susceptibility to metal-related artifacts.3 Therefore, overall, MRI is the preferred imaging technique for the assessment of pulmonary venous anomalies, although the better anatomic detail of CT could prove decisive when choosing which exam to use. Although the standard technique for the assessment of these anomalies has long been cardiac catheterization, they can also be accurately assessed by MRI or CT, and so this invasive exam is frequently unnecessary.
TreatmentUnlike PAPVC, no catheter-corrective treatment is possible for TAPVC, although atrial septostomy is used in some patients when corrective surgery is delayed. In all cases, the goal of surgery is to redirect pulmonary vein flow entirely to the left atrium and to repair associated anomalies. TAPVC is still associated with significant morbidity and mortality, due to the severe hemodynamic and metabolic compromise at presentation. Moreover, 10-15% of patients undergoing repair of TAPVC require multiple interventions due to recurrent stenosis after initial successful correction, with an increasingly poor outcome at each representation.8
In pediatric patients, PAPVC is usually treated if they have Qp:Qs of 1:1.5 or more as they are more likely to develop pulmonary hypertension and right ventricular failure,9 although this cutoff has not been subject to rigorous study. In adult patients, the criteria for surgical repair are less clear cut. Those who have already developed symptoms due to shunting, or have evidence of right-sided volume overload, can be considered for surgery. However, in asymptomatic patients with a low shunt fraction and no clinical or evidence of right heart overload or pulmonary hypertension, surgery may be unnecessary.
In cases of PAPVC, there are a number of correction procedures, depending on the number and the site of the anomalous vein or veins. Among several techniques, the Warden method or modified Warden method are usually used. The optimal time for intervention is preschool age. A European Congenital Heart Surgeons Association multicenter study aimed to analyze the surgical results and outcomes of patients who underwent surgery for scimitar syndrome, either by baffling the anomalous drainage into the left atrium via a tunnel or transecting the ‘scimitar drainage’ near its entrance into the inferior vena cava and then reimplanting it directly into the left atrium.10 Their analysis demonstrated that corrective surgery can be done safely with low mortality and morbidity, independently of the type of surgical technique used, especially if conducted prior to the development of pulmonary hypertension.
Percutaneous transcatheter occlusion of an anomalous pulmonary venous connection using coils or an Amplatzer occlusion device has also been reported, although this is only feasible when the anomalous pulmonary veins connect to both the left atrium and the systemic veins.11
Thus, for patients with PAPVC who have already developed mild to moderate pulmonary hypertension, surgical repair is usually safe and effective, although catheter-guided and medical therapies may play an increasing role. Finally, in patients who have already progressed to severe pulmonary hypertension, lung or heart-lung transplantation may be necessary.
ConclusionThere is a wide spectrum of pulmonary venous developmental anomalies. Their exact drainage pattern and associated complications can be identified by different imaging techniques. CT and MRI are noninvasive imaging techniques that play an increasingly important role in the assessment of these patients.
Most patients with an anomalous pulmonary venous connection are treated surgically if they are symptomatic or if they have significant left-to-right shunting. Percutaneous treatment is occasionally possible in PAPVC. Physicians who diagnose and treat adult patients with pulmonary hypertension, right chamber dilatation with signs of volume overload and an intact atrial septum should always consider abnormal venous pulmonary drainage as a potential etiology.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.