Valvular heart disease is common in systemic lupus erythematosus (SLE) and antiphospholipid syndrome. Immunologic insult plays a fundamental role in its pathogenesis but data on the role of antiphospholipid antibodies have been inconsistent, particularly regarding SLE-associated valvular lesions. Although timely diagnosis is essential to prevent progression of valvular lesions, treatment remains a challenge because of the lack of large systematic studies. This article reviews and summarizes recent information relating to valvular damage in these two autoimmune diseases, and highlights some important questions that need to be answered.
É elevada a prevalência da doença valvular no lupus eritematoso sistémico e na síndrome antifofolipideo. A lesão imunológica tem um papel primordial no desenvolvimento da doença valvular mas os estudos que suportam o papel dos anticorpos antifosfolipideo na sua patogénese tem sido inconsistente. Apesar do diagnóstico atempado e precoce ser essencial na prevenção da progressão das lesões valvulares, a estratégia terapêutica continua a ser um desafio importante devido à falta de grandes estudos multicêntricos. Esta revisão pretende rever e sumariar toda a informação recente relacionada com a lesão valvular associada a estas doenças auto-imunes bem como alertar os leitores para algumas questões que ainda não têm uma resposta imediata.
The cardiovascular system is a frequent and characteristic target of several systemic autoimmune diseases, in particular systemic lupus erythematosus (SLE), a disorder that results in inflammatory damage in multiple organs. Libman–Sacks endocarditis was first described in 1924 by Emanuel Libman and Benjamin Sacks, who characterized this nonbacterial verrucous valvular disease in four patients with SLE.1 Improvements in diagnostic techniques and better treatment with prolonged survival provide evidence that cardiac involvement can be potentially severe in SLE.2,3
The link between antiphospholipid antibodies (aPL) and Libman–Sacks endocarditis was first recognized in a case report published in 1985 by D’Alton et al., who reported a woman with SLE, positive lupus anticoagulant and verrucous endocarditis.4
Antiphospholipid syndrome (APS) or Hughes syndrome was first described in 1983 by Graham Hughes as anticardiolipin syndrome,5 and is characterized by venous and/or arterial thrombosis, recurrent pregnancy loss and the presence of aPL. The term anticardiolipin syndrome was replaced by the term antiphospholipid syndrome when it became clear that antibodies against phospholipids other than cardiolipin were also associated with clinical manifestations of APS. This syndrome can be either primary or secondary to an underlying condition (most commonly SLE).6–10
Although typically mild and asymptomatic, Libman–Sacks endocarditis can lead to significant morbidity, being associated with serious complications such as superimposed bacterial endocarditis, thromboembolic events and severe valvular regurgitation and/or stenosis requiring surgery.3
EpidemiologyCardiovascular complications have long been recognized as an important cause of morbidity and mortality in autoimmune disease. Valvular thickening is frequent in SLE patients, and valve vegetations in autopsy studies have been observed in 30–50%; however, symptomatic valvular disease with hemodynamic implications is rare.3,10–12 To determine the real prevalence of Libman–Sacks endocarditis, several echocardiographic studies have been performed in recent years, some of which assessed the relationship between valvular lesions and aPL.
In the first prospective echocardiographic study, published in 1988, Galve et al. found clinically significant valvular lesions in 18% of patients with SLE (the prevalence of Libman–Sacks endocarditis was 9.5%).13 Two years later Nihoyannopoulos et al. found an overall prevalence of cardiac involvement of 58%. The most common pathology was valvular (28%), ranging from thickened leaflets with impaired valve function (20%) to vegetations (9%), followed by pericardial involvement (21%) and myocardial dysfunction. The same report showed that high levels of anticardiolipin antibodies were strongly associated (p<0.0001) with cardiac abnormalities, not only in SLE but also in other lupus-like syndromes.14 Several other recent studies have analyzed the role of aPL. Some authors found an association between valvular abnormalities detected by transthoracic echocardiography and the presence of aPL.14–18 However, other authors, who used transesophageal echocardiography, found no differences in the prevalence of valvular disease between patients with and without aPL.19
To clarify the relationship in patients with SLE between aPL and incidence and progression of severe valvular dysfunction requiring valve replacement, a Spanish research group recently published a prospective cohort study that enrolled 61 patients and 40 matched controls during 14±3 years of follow-up. This long-term study found an increase of 39–73% in valvular abnormalities, but only 12% of patients developed severe valvular regurgitation. Until the publication of this study, attempts to identify patients at risk of developing severe valvular dysfunction had been unsuccessful. This group demonstrated that severe valvular regurgitation was significantly associated with the presence of high levels of IgG anticardiolipin antibodies (p=0.001).20 Another prospective cohort study that enrolled a significantly higher number of patients (n=342) with a shorter follow-up period (four years) and with the same imaging modality (transthoracic echocardiography) determined the prevalence and progression of Libman–Sacks endocarditis. Of the 38 patients (11%) with Libman–Sacks endocarditis, nine progressed to more severe lesions during the follow-up period and eight new cases were diagnosed. This study reported a significant association between Libman–Sacks endocarditis and disease duration, activity, thrombosis, stroke, thrombocytopenia, anticardiolipin antibodies and APS.21
Pathogenesis of valvular lesions in SLE and APSThe pathogenesis of Libman–Sacks endocarditis is still unclear. The initial insult may be immunologic, as suggested by the presence of immunoglobulins and complement on affected valves. The deposition of fibrin-platelet thrombi onto the injured valve results in valve fibrosis, edema, diffuse thickening, mild inflammatory changes, valve distortion, scarring, and consequently valvular dysfunction.22–24 Although there are no pathognomonic microscopic findings, it is common to see fibrin deposits, neovascularization, hyalinosis, calcinosis and a variable extent of inflammatory cell infiltration with mononuclear predominance. The end-stage of Libman–Sacks verrucous endocarditis can become a fibrous plaque sometimes showing focal calcification.2,3,26 In this process the deposition of aPL under valves compromised by immune complex deposition can foster thrombus formation and inflammatory processes.3,21–25 In a multicenter study Vianna et al. showed a significantly higher prevalence of valvular lesions in patients with APS secondary to SLE than in those with primary APS. This suggests that additional SLE factors involved in endocardial damage may play a specific pathogenetic role.18 This process seems to be a continuous one that commences with immunologic lesions, evolves into valvular thickening and culminates with the formation of vegetations and their clinical consequences.
DiagnosisValvular lesions are frequent in patients with SLE and APS, but clinically significant valvulopathy occurs in only a small proportion in the form of heart failure or Libman–Sacks endocarditis. The latter, also known as verrucous endocarditis, is the end-stage of a progressive process and is typically asymptomatic, but when symptoms are present the clinical syndrome mimics infective endocarditis, so diagnosis can be difficult. In intravenous drug users infective endocarditis and Libman–Sacks endocarditis can coexist,11,27 and it is imperative to differentiate between these two medical conditions since their management and treatment differ.
To distinguish Libman–Sacks endocarditis from infective endocarditis, the modified Duke criteria may be helpful. The main Duke criteria include the demonstration of typical microorganisms from two separate blood cultures (or one positive blood culture for Coxiella burnetii or phase I IgG antibody titer >1/800), or evidence of endocardial involvement by echocardiogram. The minor Duke criteria include fever >38.0°C, vascular phenomena (major arterial emboli, septic pulmonary infarcts, mycotic aneurysm, intracranial hemorrhages, conjunctival hemorrhages, Janeway lesions), immunologic phenomena (glomerulonephritis, Osler nodes, Roth spots, rheumatoid factor), microbiologic evidence (with blood cultures not meeting major criteria or serologic evidence of active infection with an organism consistent with infectious endocarditis) or predisposing conditions such as prior heart conditions or intravenous drug use. The diagnosis of bacterial endocarditis is definitively established with two major criteria, one major criterion and three minor criteria, or five minor criteria. A possible diagnosis is established by one major and one minor criterion or three minor criteria.28,29
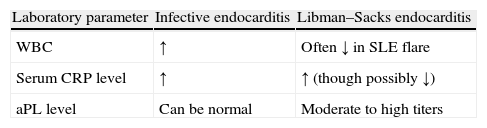
Ménard et al., in a case report published in 2008, described a 43-year-old woman with Libman–Sacks endocarditis and defined some characteristics favoring a diagnosis of this disease. In addition to blood cultures they identified three useful laboratory tests (Table 1). White blood cell count is frequently low during a lupus flare but high in infective endocarditis. C-reactive protein level is elevated in SLE (as expected for an inflammatory disease), but very high levels favor infective endocarditis. Levels of aPL, if moderately to highly positive, suggest SLE.22,31 As previously shown, another important and difficult differential diagnosis is with intracardiac tumors such as papillary fibroelastoma. Echocardiography can play a very useful role in this challenging situation.32–35
Helpful marker in distinguishing infective endocarditis from Libman–Sacks endocarditis.
| Laboratory parameter | Infective endocarditis | Libman–Sacks endocarditis |
| WBC | ↑ | Often ↓ in SLE flare |
| Serum CRP level | ↑ | ↑ (though possibly ↓) |
| aPL level | Can be normal | Moderate to high titers |
aPL: antiphospholipid antibodies; CRP: C-reactive protein; SLE: systemic lupus erythematosus; WBC: white blood cell count. Adapted from Ref. 30.
Echocardiography appears to be the best imaging modality to diagnose valvular disease. As noted above, echocardiography plays a major role in diagnosing Libman–Sacks endocarditis and differentiating between this entity (and other valvular lesions associated with SLE and APS) and infective endocarditis or intracardiac tumors. Below we describe the echocardiographic characteristics that can help in the differential diagnosis.
Echocardiographic features of Libman–Sacks endocarditisEchocardiographically, Libman–Sacks vegetations appear as valve masses of varying size and shape, generally more than 2mm in diameter. Their borders are frequently irregular and they are firmly attached to the valve surface. These vegetations are usually sessile and exhibit no independent motion.21 Occasionally, Libman–Sacks lesions occur at the commissures, free margins and valve rings, but are more frequent on the atrial or ventricular valve surface. Left-sided heart valves are more often affected. When the mitral valve leaflet is involved, vegetations may extend to the subvalvular apparatus (chordae tendineae, papillary muscles) and the adjacent mural endocardium.2,22,36 Perez-Villa et al., among others, showed that valvular regurgitation is the most common abnormality: mitral regurgitation was seen in 26% of patients, aortic regurgitation in 7% and tricuspid regurgitation in 7%.20 The most frequent valvular abnormality in several studies was valvular thickening. Transesophageal echocardiography is more accurate in detecting Libman–Sacks endocarditis than transthoracic echocardiography, as demonstrated in the first prospective randomized controlled trial, by Roldan et al.37
Echocardiographic features of infective endocarditisInfective vegetations tend to be located nearer to the leaflet line of closure than Libman–Sacks vegetations. In addition, the former exhibit independent motion and have a homogeneous echodensity on echocardiography.30,38,42
TreatmentMost publications do not distinguish clearly between different types of valvular involvement, such as valvulitis (potentially reversible thickening), valve deformity or verrucous vegetations (Libman–Sacks endocarditis). This makes it difficult to draw conclusions about the efficacy of different therapeutic options. Immunosuppressive therapy – particularly with corticosteroids – is the cornerstone of treatment for SLE, but although these drugs facilitate gradual healing of the lesions by decreasing inflammation, they also promote fibrosis and scarring, resulting in additional valvular damage.22,39,40 Nevertheless, corticosteroids are essential to control disease activity. When Libman–Sacks endocarditis is found in an early active stage, corticosteroids (prednisone 1mg/kg/day) are recommended.41 Although the use of steroids to treat SLE valvular lesions is relatively consensual, this treatment is not recommended for primary APS. Nesher et al., in 1997, advocated the use of corticoids to improve valve function in patients with severe mitral regurgitation secondary to primary APS.43 In contrast, other studies found no benefit and advocated only anticoagulation and/or antiplatelet therapy. To date, however, no systematic studies have appeared on immunosuppressive or anti-inflammatory treatments of valve disease in these patients.
Surgical treatmentSevere valvular disease requiring surgical treatment in patients with APS and/or SLE is rare. However, when severe valvular dysfunction cannot be controlled with conservative treatment, valve surgery may be required. A careful choice of surgical procedure, i.e., valve repair or replacement with a mechanical valve or a bioprosthesis, is extremely important. Each procedure has advantages and disadvantages. Although real consensus is lacking, most authors have argued in favor of mechanical valve replacement or valve repair for patients with SLE.44 However, in 2010, Colli et al., in a retrospective study with a median follow-up of 66 months, analyzed nine patients with APS treated with heart valve surgery (six mechanical valves, two tissue heart valves and one plasty). They found high morbidity (50%) and mortality (22%) related to thrombotic or hemorrhagic complications. Although there are some theoretical reasons to prefer mechanical prostheses, the follow-up results in these patients are discouraging, mainly related to anticoagulation treatment and to the patients’ hypercoagulable state, which may cause immunologic deterioration of tissue heart valves. These authors therefore concluded that their general strategy needed to be reconsidered, and proposed that tissue heart valve prostheses could be a suitable substitute because they minimize the risks of morbidity and mortality due to hypercoagulable states in APS.45 More studies with larger populations and longer follow-up periods are needed to shed more light on this question.
Prophylaxis for bacterial endocarditisAnother practical issue is the role of antimicrobial therapy to prevent bacterial endocarditis in patients with SLE or APS. In view of the lack of consensus, Lockhart et al. reviewed the available evidence regarding the use of prophylactic antibiotics in dental practice. The authors selected eight groups of patients with specific medical conditions, including SLE, and concluded that antimicrobial prophylaxis was not advisable. They categorized SLE as a class III condition, i.e. one “for which there is evidence and/or general agreement that the procedure or treatment is not useful or effective, and in some cases may be harmful” and noted that the evidence should be considered level C, i.e., “only consensus opinion of experts, case studies or standard of care”.44,45 In 2005 Dória et al. emphasized the value of antimicrobial prophylaxis before dental treatment, especially in patients being treated with immunosuppressants, but their findings do not support this therapeutic strategy.46 The European Society of Cardiology guidelines for infective endocarditis, revised in 2009, do not advise antimicrobial prophylaxis in patients with SLE or APS.47
ConclusionsIn patients with SLE or APS, valvular lesions are the most common type of cardiac abnormality, and can result in significant morbidity and mortality. The prevalence of Libman–Sacks endocarditis has been underestimated because it is often asymptomatic or presents with only mild clinical signs and symptoms. Some of the differences in prevalence reported in different studies are related to the classification methodology. Valvular lesions associated with SLE and APS can occur as a spectrum from thickening to valve masses (nonbacterial vegetations). Another issue concerns the imaging modality used. The higher prevalences of valvular lesions in more recent studies may be related not only to better survival but also to the use of improved imaging methods.
Cardiovascular involvement in SLE and APS continues to be a leading cause of death, and this makes it essential for clinicians to recognize the typical presentation of Libman–Sacks endocarditis and its complications. Regardless of the differing views on the relationship between valvular lesions and disease activity, adequate control of the immune disease is essential to prevent other sequelae. Relevant issues that await clarification in future research include the indications for prophylaxis of infective endocarditis in these patients, the role of anticoagulation in valvular lesions associated with SLE, the use of corticoids to improve valve function in APS, and the best surgical procedure for severe valvular disease.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank K. Shashok for improving the use of English in the manuscript.