To assess whether contractile reserve during dobutamine stress echocardiography (DSE) can predict left ventricular functional recovery in patients with peripartum cardiomyopathy and to assess myocardial fibrosis by magnetic resonance imaging (MRI) in these patients.
MethodsNine patients with peripartum cardiomyopathy were enrolled. All patients underwent DSE and were followed for six months, when a rest Doppler echocardiogram was repeated. MRI was also performed at the beginning of follow-up to identify myocardial fibrosis.
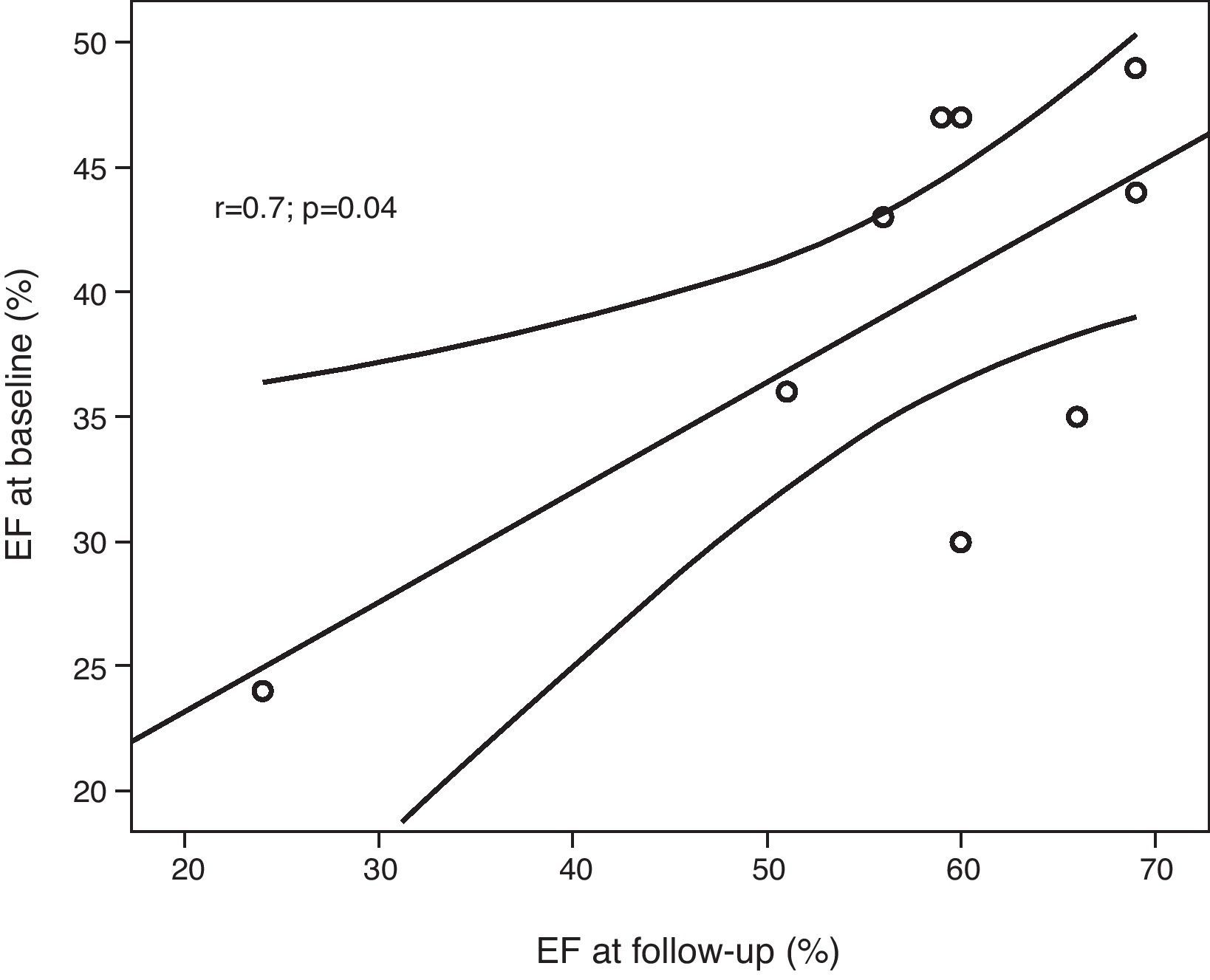
ResultsMean age was 29±7.9 years and mean left ventricular ejection fraction at baseline was 39.4±8.6% (range 24–49%). Eight of the nine patients showed left ventricular functional recovery with mean ejection fraction at follow-up of 57.1±13.8%. The ejection fraction response to DSE did not predict recovery at follow-up. On the other hand, left ventricular ejection fraction at baseline correlated with ejection fraction at follow-up. Mild fibrosis was detected in only one patient.
ConclusionLeft ventricular ejection fraction at baseline was a predictor of left ventricular functional recovery in patients with peripartum cardiomyopathy. Dobutamine stress echocardiography at presentation of the disease did not predict recovery at follow-up. Myocardial fibrosis appeared to be uncommon in this cardiomyopathy.
Avaliar se a reserva contrátil durante o ecocardiograma de estresse com dobutamina (EED) pode predizer a recuperação funcional do ventrículo esquerdo em pacientes com miocardiopatia periparto e também acessar a fibrose miocárdica através da ressonância nuclear magnética (RNM) nestas pacientes.
MétodosNove pacientes com miocardiopatia periparto foram incluídas. Todas as pacientes foram submetidas ao EED e acompanhadas por 6 meses, quando um novo ecocardiograma de repouso foi realizado. A RNM também foi realizada no início do seguimento para identificar fibrose miocárdica.
ResultadosA idade média das pacientes foi de 29±7,9 anos e a fração de ejeção basal média do ventrículo esquerdo foi de 39,4±8,6% (variando de 24 a 49%). Oito das nove pacientes tiveram recuperação funcional do ventrículo esquerdo, com fração de ejeção média no seguimento de 57,1±13,8%. A resposta da fração de ejeção ao EED não foi um preditor de recuperação no seguimento. Por outro lado, a fração de ejeção basal teve correlação com a fração de ejeção no seguimento. Fibrose discreta foi detectada em apenas uma paciente.
ConclusãoA fração de ejeção basal do ventrículo esquerdo foi um preditor de recuperação funcional ventricular em pacientes com miocardiopatia periparto. O EED na apresentação da doença não foi um preditor de recuperação no seguimento. Fibrose miocárdica pareceu ser incomum nesta miocardiopatia.
Peripartum cardiomyopathy (PC) is a rare disease, recognized as early as the 18th century,1 and its diagnostic criteria were established in 1937.2,3 It is characterized by heart failure during the last month of pregnancy through the fifth month postpartum, without heart disease before the last gestational month, and no determinable cause.3
Because of its rarity, geographical differences and heterogeneous presentation, diagnosis may be difficult.3 Traditionally, it has been related to old maternal age, black race, greater parity and multiple gestation, but the underlying cause remains elusive.4 The reported prevalence of this disorder ranges from one in 100 to one in 15000 pregnancies.2,5,6 In a recent report, the incidence in 241497 deliveries was one in 4025, and was highest among African-Americans.7
In contrast to idiopathic dilated cardiomyopathy, left ventricular (LV) dilation and systolic dysfunction return to normal in more than 50% of patients within six months,2 although in a recent study in 100 African women with PC, the authors reported that ejection fraction (EF) returned to normal in only 23% of the patients.8 Higher EF and smaller LV diameter at the time of diagnosis have been shown to be associated with recovery and with a better prognosis,4,8–10 although there is some controversy.11 Although the presence of fibrosis, detected by biopsy12 and by cardiac magnetic resonance imaging (MRI),13,14 has been described in PC, its role in recovery of function is not known.
Dobutamine is a synthetic sympathomimetic amine that directly stimulates beta-1 receptors in the myocardium to increase myocardial contractility. Dobutamine stress echocardiography has been shown to be safe and accurate in detecting coronary artery disease15 and evaluating myocardial viability in patients with LV dysfunction.16 More recently, it has been used to analyze contractile reserve in patients with PC in order to predict recovery of function.17
The objectives of the present study were: (1) to assess whether low-dose dobutamine stress echocardiography, performed at an early stage of PC, can predict recovery of LV function in these patients; and (2) to assess whether myocardial fibrosis can be detected by cardiac magnetic resonance imaging (MRI).
MethodsStudy groupNine consecutive women with a diagnosis of PC from a single public maternity hospital were enrolled. Patients were included only if they were seen by the cardiologist in the first week after they had sought medical assistance. The diagnosis of PC was based on the development of congestive heart failure during pregnancy or the puerperium, if previous heart diseases or possible precipitating factors (anemia, morbid obesity, cesarean section myocarditis, infection, alcohol abuse) could be excluded.
Doppler echocardiogramDuring the development of the disease, all patients underwent a comprehensive Doppler echocardiogram which detected some degree of systolic dysfunction (EF <50% during optimized medical treatment for congestive heart failure) soon after delivery, to confirm the diagnosis of systolic dysfunction. The exams were performed by the same experienced echocardiographer using a Philips 5500 system (Philips Medical Systems, N.A., Bothell, WA). Measurements were made according to the ASE recommendations18 and systolic and diastolic function were analyzed. The diastolic filling pattern was categorized as stage I (abnormal relaxation), stage II (pseudonormal) or stage III (restrictive pattern), by a combination of transmitral and pulmonary vein flows, as well as tissue Doppler, as previously validated.19,20 Since there were no wall motion abnormalities, EF was measured by the Teichholz method. Patients then underwent a low-dose dobutamine stress echocardiogram and a rest Doppler echocardiogram was repeated after six months.
Written informed consent was obtained from all patients, as well as the consent of the patient's attending cardiologist. The study protocol was approved by the Ethics Committee of our institution.
Dobutamine stress echocardiogramDSE was performed in all patients in the postpartum period, when patients were already receiving full treatment for congestive heart failure. In all cases the exam was performed in the first three days after patients were seen by a cardiologist, although some had had symptoms for more than a month (3–60 days) before they sought medical assistance. DSE was performed by the same cardiologist. Dobutamine infusion started at 5μg/kg/m and was increased every 3minutes by 5μg/kg/m until a dose of 20μg/kg/m was reached. Heart rate, blood pressure, and ECG were monitored throughout the exam. LV diameters and EF were obtained at each stage.
Magnetic resonance imagingMRI was performed on a 1.5-T GE Signa LX system (GE Medical Systems, Waukesha, Wisconsin) during follow-up to assess the presence of myocardial fibrosis after the intravenous administration of gadolinium chelate by the late-enhancement technique.21
Follow-upSix months after delivery, a rest Doppler echocardiogram was obtained by the same cardiologist, and the same measurements were performed to analyze systolic and diastolic function. The cardiologist performing the exam was unaware of the DSE data. Patients continued to be followed to detect any heart failure complications but none occurred. All but one patient were still on congestive heart failure medication.
Statistical analysisCategorical data are presented as absolute values and percentages, and continuous data are expressed as mean values±SD. The significance of baseline differences was determined by the chi-square test, Fisher's exact test, or the unpaired t-test, as appropriate.
Pearson's correlation coefficient was used to measure the correlation between EF at baseline, during stress echocardiography and at follow-up. A p value of <0.05 was established as statistically significant. SPSS version 13 (SPSS Inc., Chicago, IL) was used for all analyses.
ResultsNine women were studied. Mean age was 29.7±7.9 (range 16–38). All patients began to have symptoms of heart failure either prepartum (three patients, initial presentation: 3, 7 and 30 days before delivery) or postpartum (six patients, initial presentation: 4, 5, 9, 10, 12, and 30 days after delivery). Two presented with acute pulmonary edema, one with stroke and one with pulmonary embolism. Although four patients had a diagnosis of pre-eclampsia (44%), they had no previous history of heart failure, so the diagnosis of peripartum cardiomyopathy could be made.3,6 One patient had sickle cell trait.
At the time of DSE, all patients were on angiotensin-converting enzyme (ACE) inhibitors and diuretics. Three patients were on anticoagulants, two on beta-blockers and two on digoxin. There were no neonatal deaths.
ElectrocardiogramECGs were obtained in all patients. A normal ECG was present in only two patients. One patient had sinus bradycardia due to the use of propranolol. Three had sinus tachycardia, two with associated ST-T abnormalities and one of these with LV hypertrophy. Another patient had left atrial hypertrophy and right bundle branch block and two patients had sinus rhythm and mild ST-T abnormalities.
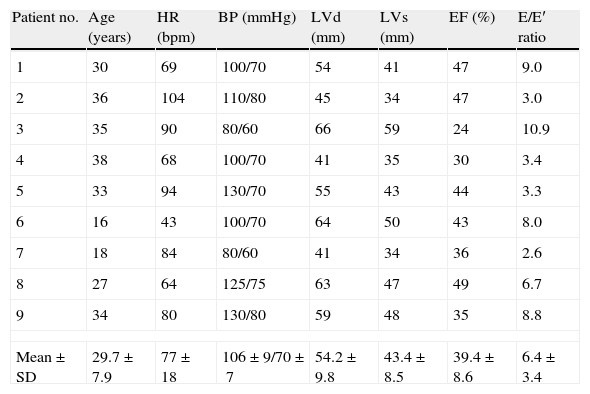
Doppler echocardiogram at rest and during DSEBaseline parameters are presented in Table 1. Mean EF at baseline was 39.4±8.6%. Three patients had EF ≤35%, three had EF ≤45% and three had EF >45% and <50%. All patients with EF >45% and <50% at the time of the exam had had EF <45% at diagnosis.
Clinical characteristics and Doppler echocardiographic parameters in nine patients with peripartum cardiomyopathy.
| Patient no. | Age (years) | HR (bpm) | BP (mmHg) | LVd (mm) | LVs (mm) | EF (%) | E/E′ ratio |
| 1 | 30 | 69 | 100/70 | 54 | 41 | 47 | 9.0 |
| 2 | 36 | 104 | 110/80 | 45 | 34 | 47 | 3.0 |
| 3 | 35 | 90 | 80/60 | 66 | 59 | 24 | 10.9 |
| 4 | 38 | 68 | 100/70 | 41 | 35 | 30 | 3.4 |
| 5 | 33 | 94 | 130/70 | 55 | 43 | 44 | 3.3 |
| 6 | 16 | 43 | 100/70 | 64 | 50 | 43 | 8.0 |
| 7 | 18 | 84 | 80/60 | 41 | 34 | 36 | 2.6 |
| 8 | 27 | 64 | 125/75 | 63 | 47 | 49 | 6.7 |
| 9 | 34 | 80 | 130/80 | 59 | 48 | 35 | 8.8 |
| Mean±SD | 29.7±7.9 | 77±18 | 106±9/70±7 | 54.2±9.8 | 43.4±8.5 | 39.4±8.6 | 6.4±3.4 |
BP: blood pressure; bpm: beats per minute; E/E′: ratio of the mitral valve E wave to the mitral annular velocity E′; EF: ejection fraction; HR: heart rate; LVd: left ventricular diastolic diameter; LVs: left ventricular systolic diameter.
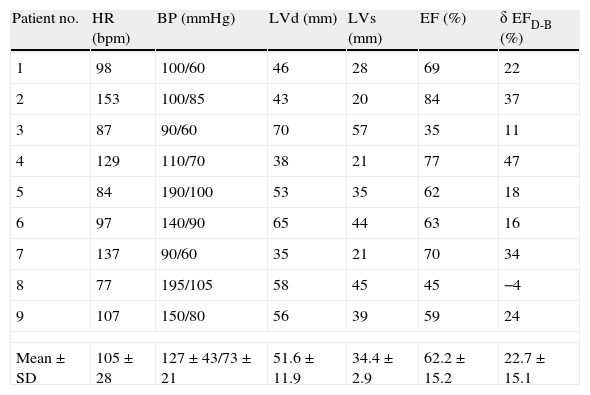
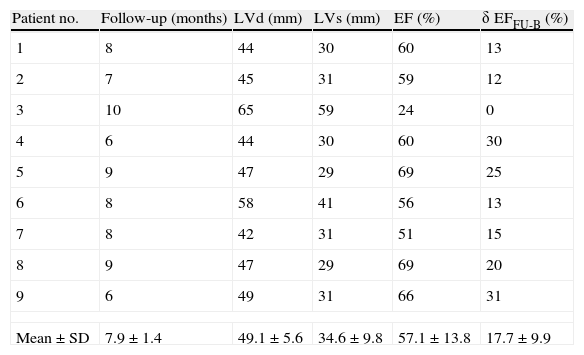
Parameters at 20μg/kg/m (or at 15μg/kg/m, when this dose showed higher EF) are presented in Table 2. Mean EF at 15 or 20μg/kg/m was 62.2±15.2%. Table 3 shows parameters at follow-up (mean EF at follow-up was 57.1±13.8%).
Doppler echocardiographic parameters at maximal contractility during dobutamine in nine patients with peripartum cardiomyopathy.
| Patient no. | HR (bpm) | BP (mmHg) | LVd (mm) | LVs (mm) | EF (%) | δ EFD-B (%) |
| 1 | 98 | 100/60 | 46 | 28 | 69 | 22 |
| 2 | 153 | 100/85 | 43 | 20 | 84 | 37 |
| 3 | 87 | 90/60 | 70 | 57 | 35 | 11 |
| 4 | 129 | 110/70 | 38 | 21 | 77 | 47 |
| 5 | 84 | 190/100 | 53 | 35 | 62 | 18 |
| 6 | 97 | 140/90 | 65 | 44 | 63 | 16 |
| 7 | 137 | 90/60 | 35 | 21 | 70 | 34 |
| 8 | 77 | 195/105 | 58 | 45 | 45 | −4 |
| 9 | 107 | 150/80 | 56 | 39 | 59 | 24 |
| Mean±SD | 105±28 | 127±43/73±21 | 51.6±11.9 | 34.4±2.9 | 62.2±15.2 | 22.7±15.1 |
BP: blood pressure; bpm: beats per minute; EF: ejection fraction; δ EFD-B: variation of EF from 20μg/kg/min dobutamine to baseline; HR: heart rate; LVd: left ventricular diastolic diameter; LVs: left ventricular systolic diameter.
Doppler echocardiographic parameters at follow-up in nine patients with peripartum cardiomyopathy.
| Patient no. | Follow-up (months) | LVd (mm) | LVs (mm) | EF (%) | δ EFFU-B (%) |
| 1 | 8 | 44 | 30 | 60 | 13 |
| 2 | 7 | 45 | 31 | 59 | 12 |
| 3 | 10 | 65 | 59 | 24 | 0 |
| 4 | 6 | 44 | 30 | 60 | 30 |
| 5 | 9 | 47 | 29 | 69 | 25 |
| 6 | 8 | 58 | 41 | 56 | 13 |
| 7 | 8 | 42 | 31 | 51 | 15 |
| 8 | 9 | 47 | 29 | 69 | 20 |
| 9 | 6 | 49 | 31 | 66 | 31 |
| Mean±SD | 7.9±1.4 | 49.1±5.6 | 34.6±9.8 | 57.1±13.8 | 17.7±9.9 |
EF: ejection fraction; δ EFFU-B: variation of EF from follow-up to baseline; LVd: left ventricular diastolic diameter; LVs: left ventricular systolic diameter.
With the use of dobutamine, all patients but one (patient 8) showed contractile reserve with increased EF. All patients were stable and normotensive at the time DSE was performed. Two patients developed mild hypertension, as commonly seen with dobutamine stress echocardiography, but this was not accompanied by symptoms and regressed rapidly after the exam. No significant side effects were observed during DSE.
EF had normalized in seven patients at follow-up. Patient 3's EF remained low and in patient 7, though an increase in EF was seen from 36% at baseline to 51% at follow-up, mild systolic dysfunction persisted.
At baseline, E/E′ ranged from 2.6 to 12.2 (mean 6.4±3.4) and was above 10 only in a patient with stage III diastolic dysfunction and severe systolic dysfunction. Seven patients were in stage I diastolic dysfunction. Only patient 3 showed stage III diastolic dysfunction. Interestingly, this was the only patient who did not show any improvement in EF at follow-up. In one patient (patient 9), diastole was normal by all Doppler echocardiographic parameters (she was under optimized treatment for heart failure).
Follow-upPatients underwent a rest Doppler echocardiogram after 6 months of the clinical diagnosis of peripartum cardiomyopathy (mean follow-up 7.9±1.4 months, range 6–10). All patients, including the one in whom low EF persisted, were in class I or II, although the majority were still on medication (six patients were on ACE inhibitors, four on diuretics, two on beta-blockers and one on digoxin). One patient had pulmonary embolism a week after delivery and another had a cerebral stroke a month after delivery. No patient died.
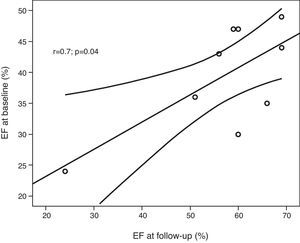
Eight patients had an increase in EF, which normalized in seven patients. EF increased by a mean of 17.7±9.9% from baseline to follow-up (range 0–31%), mild systolic dysfunction persisting in one patient (patient 7, EF=51%) and severe dysfunction in another (patient 3, EF=24%). There was a strong correlation between EF at baseline and at follow-up (r=0.699, p=0.036) (Fig. 1).
MRISeven of the nine patients (77%) underwent cardiac MRI 16.3±11.3 months after the diagnosis of PC (2–29 months). Myocardial fibrosis was detected in only one patient (14%), who showed minimal midwall fibrosis in the mid segment of the LV anterior wall (patient 1). Her MRI was performed eight months after the diagnosis and her EF at the time of enrollment was 47%. The presence of myocardial fibrosis did not correlate with EF at baseline, nor with its increase with dobutamine or with EF at follow-up in this patient.
DiscussionThe present study showed that EF at clinical onset of peripartum cardiomyopathy correlates with improvement in systolic function after six months. Although this has been previously suggested,22 several studies failed to demonstrate correlation of either LV size or LV function with outcome in peripartum cardiomyopathy.11,23,24
Similarly to our study, Fett et al. reported that LV echocardiographic features at diagnosis were unable to predict individually who would recover. They studied 92 patients in Haiti who were followed for five years and showed that LV echocardiographic features at diagnosis were not predictive of individual recovery. However, EF at diagnosis differed statistically between recovered and nonrecovered groups (28% vs. 23%, p<0.001).25 This is similar to what we observed in our study, in which a statistically significant difference in mean EF at diagnosis was seen between recovered and nonrecovered patients.
Although the US National Heart, Lung, and Blood Institute defines peripartum cardiomyopathy4 as heart failure within the last month of pregnancy or five months postpartum; absence of preexisting heart disease; no determinable etiology (the traditional definition) together with strict echocardiographic criteria of LV dysfunction: EF less than 45%, or M-mode fractional shortening less than 30%, or both, and end-diastolic dimension more than 2.7cm/m2, a cut-off value of 50% for EF was used in the present study. The reason for a higher cut-off value was that stress echocardiography was performed only when patients were stable and already receiving optimized treatment for congestive heart failure. However, all patients had objective signs and symptoms of congestive heart failure, appearing either in late pregnancy or in the puerperium (two patients had had acute pulmonary edema at presentation), and those who had EF >45% and <50% all had EF <45% at admission.
Six patients (67%) had mild or moderate systolic dysfunction at the time of stress echocardiography. The fact that EF was only mild or moderately reduced in six of these nine patients may be one of the reasons that eight of them improved at follow-up. In fact, the only patient in whom severe LV systolic dysfunction persisted had severe LV dysfunction at presentation. Witlin et al. demonstrated that women with severe myocardial dysfunction (LV end-diastolic dimension ≥60mm and fractional shortening ≤21%) resulting from peripartum cardiomyopathy are unlikely to regain normal cardiac function on follow-up echocardiographic study (severe dysfunction persisted in six out of seven), while in two of their patients with mild dysfunction, mild dysfunction persisted in one and the other recovered fully.22
Our finding of normalization of systolic function in seven of nine patients (78%) is in contrast to what has previously been reported, but supports more recent findings of a higher incidence of recovery of systolic function (around 75%) and better prognosis in patients with PC compared to other forms of dilated cardiomyopathy.26,27 The small number of patients with mild systolic dysfunction at the time of DSE may be one of the reasons that LV functional recovery occurred in the majority of our patients.
As in other studies, there was a history of hypertension during pregnancy in 44% of our patients, and this diagnosis should not exclude the diagnosis of peripartum cardiomyopathy.3
Lampert et al. used low-dose dobutamine stress echocardiography to demonstrate that nonpregnant women who had recovered from postpartum cardiomyopathy had lower contractility reserve than matched controls. The change in LV contractility over baseline values was significantly less in women with a normal rest echocardiogram who had recovered from peripartum cardiomyopathy than in a group of normal matched control subjects challenged in an identical manner. Although patients appear to recover clinically and by rest echocardiogram, there may be some residual subclinical abnormalities that can only be detected when the myocardium is subjected to a significant hemodynamic stress, such as the use of dobutamine stress echocardiography. The authors speculated that contractile reserve in these patients could offer information regarding cardiac performance in subsequent pregnancies, but this was not investigated by their study.28
In the present study, response of EF to dobutamine stress echocardiography was not predictive of EF at follow-up. Unlike our study, Dorbala et al. showed in six patients with PC that inotropic contractile reserve during dobutamine stress echocardiography accurately correlated with subsequent recovery of LV systolic function.17 However, a p value of 0.10 was used in their study for statistically significant findings.
Dobutamine was used in doses of 5μg/kg/m in the study by Lampert et al., 28 and up to 50μg/kg/m in the study by Dorbala et al.17 A dose of 5μg/kg/m may be insufficient to detect full contractile reserve, while 50μg/kg/m is too high to analyze it. Pelicka et al. demonstrated that stroke volume during dobutamine is commonly maximum at a dose of 20μg/kg/min,29 which is why this dose was used in the present study.
The presence of fibrosis, detected by endomyocardial biopsy, has been reported in five out of 11 African women with a diagnosis of PC. A “healed” pattern of myocarditis and fibrosis was found in five patients (three out of four with persistent heart failure presented this finding).12 In another study, endomyocardial biopsy was performed in a black woman with PC and no fibrosis, necrosis or evidence of inflammation was found. These authors speculate that normal endomyocardial biopsy findings during the acute phase of the disease may be predictive of recovery.30 However, the utility of endomyocardial biopsy is unclear, with studies involving a large number of patients showing no correlation of endomyocardial fibrosis findings with prognosis.23 Other less invasive methods are thus necessary.
MRI with hyperenhancement can detect myocardial fibrosis in dilated cardiomyopathy as a midwall and focal pattern, which differs from the subendocardial fibrosis seen in myocardial infarction.21,31 Fibrosis detected by MRI has been described in four out of 10 patients (40%) with peripartum cardiomyopathy.13 It occurred in only one of seven patients (14%) in the present study, and was minimal. It may be that patients with less severe involvement of the disease were included in our group. The mean time between dobutamine stress echocardiography and MRI was 16.3±11.3 months (range 2–29 months) with one patient having had MRI only two months after delivery. Although fibrosis has been shown to decrease from the acute phase to ten months later,14 the timing for development and/or regression of fibrosis in PC is unknown and its occurrence may have been different had MRI been performed earlier on during the disease, at least in some of our patients.
Study limitationsThis is a study with a small number of patients. However, peripartum is a rare disease and the findings of this prospective evaluation of nine patients with DSE can add information on this still poorly understood disease.
Although in all cases DSE was performed one to three days after the patient was seen by a cardiologist, some patients had had symptoms for more than a month before they sought medical assistance, so by the time they underwent DSE some recovery may already have occurred.
Most of our patients had mild or moderate systolic dysfunction (EF=39.4±8.6, range 24–49%) by the time they underwent stress echocardiography, so the results may not apply to groups with more severe LV involvement.
ConclusionsIn patients with peripartum cardiomyopathy, LV ejection fraction at baseline was a predictor of LV functional recovery. Dobutamine stress echocardiography at presentation of the disease did not predict recovery at follow-up. Myocardial fibrosis as detected by cardiac MRI does not appear to be common in this disease.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank Axial and Mater Dei Hospital in Belo Horizonte, Brazil, for kindly performing the MRI exams in these patients.