Optical coherence tomography (OCT) and intravascular ultrasound (IVUS) are imaging methods used in the diagnosis of coronary lesions. IVUS is widely used in interventional cardiology laboratories, but OCT is now increasingly used. Conventional coronary angiography can identify different types of coronary lesions but sometimes is unable to diagnose them correctly. Both intravascular imaging methods are useful for better interpretation of these lesions, and can accurately diagnose ruptured plaques, thrombosis, stent restenosis and hazy images. However, the resolution of OCT is ten times higher than IVUS, and so an accurate diagnosis cannot always be achieved with ultrasound imaging. We present three cases in which IVUS was unable to identify the lesion causing the condition and OCT was required to obtain clearer images that helped to confirm the diagnosis. The advantages and disadvantages of each method are then discussed.
A tomografia de coerência ótica (TCO) e o ultrassom intravascular (USIV) são métodos de imagem geralmente utilizados nos serviços de cardiologia de intervenção. O USIV é um sistema de imagem intravascular, muito utilizado nos laboratórios de cardiologia de intervenção. A utilização de TCO começou mais tarde a ser divulgada na prática diagnóstica intracoronária. A angiografia coronária convencional pode identificar diferentes tipos de lesões coronárias que por vezes não são devidamente diagnosticadas. Os métodos de imagem são também utilizados para uma melhor interpretação destas lesões. As roturas de placas, trombose, reestenose de stent e outras imagens nebulosas podem ser diagnosticadas fielmente através da sua utilização. No entanto, a resolução da TCO é 10 vezes superior à do USIV. Portanto não é possível obter sempre um diagnóstico fiel com uma imagem ultra-som. Este artigo apresenta três casos em que a utilização de USIV não foi suficiente para identificar a lesão, tendo sido necessário recorrer à TOC para obter imagens mais claras que ajudaram a confirmar o diagnóstico. Segue-se a discussão das vantagens e desvantagens de cada método.
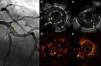
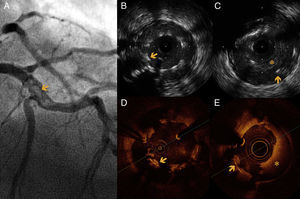
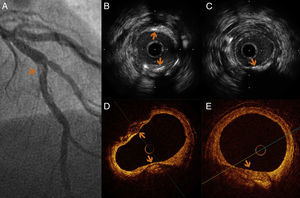
An 86-year-old woman with a history of severe three-vessel disease was treated in 2006 with a drug-eluting stent in the proximal left anterior descending artery. She was recently readmitted with an acute anterior infarction, coronary angiography showing a contrast defect in the left anterior descending artery (LAD) stent and a contrasting image outside suggestive of stent malapposition. OCT images showed extended stent malapposition, severe circumferential aneurysm with a diameter of 5 mm and abundant intraluminal thrombi. IVUS was used to better visualize the aneurysmal structure, and confirmed the lesion and thrombus image inside. Thrombus aspiration was not performed due to lack of the appropriate aspiration device. A 3.5 mm × 28 mm MGuard coated stent was implanted, with a good angiographic result (Figure 1).
(A) Coronary angiography of the left anterior descending artery (LAD) in right anterior oblique cranial view, showing an extensive intraluminal filling defect, multilobed, compatible with stent thrombosis (arrow). (B and C) Intravascular ultrasound (IVUS) images showing extensive stent strut malapposition (arrow), which appears to be coated with echogenic tissue that could correspond to adherent thrombus. (C) Extensive stent thrombosis (asterisk) and aneurysmal dilatation of the vessel outside the struts (arrow). (D and E) Optical coherence tomography (OCT) images at the same level as the previous IVUS, showing strut malapposition (arrow) with adherent platelet thrombus. (E) Extensive stent thrombosis (asterisk).
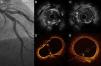
A 64-year-old man with unstable angina symptoms presented a chronic calcified lesion in the LAD on angiography. IVUS and OCT images revealed severe mixed plaque with a large calcium component occupying the entire circumference of the artery, with a luminal area of 2.5 mm2 in the distal artery and another lesion in the proximal segment with superficial calcium and a luminal area of 4 mm2.
Rotablator debulking was performed around the mid segment and in the distal artery with a 1.5-mm burr. A 2.5 mm × 20 mm balloon was expanded and two overlapping drug-eluting stents (DES), 2.5 mm × 23 mm and 3 mm × 33 mm, were implanted, with a successful result on angiography and intracoronary imaging (Figure 2).
(A) Coronary angiography of the LAD in right anterior oblique cranial view, showing a focal lesion, spiculated, with irregular edges, consistent with highly calcified atheroma (arrow). (B and C) IVUS images of the calcified lesion in the LAD. (B) Two calcified lesions characterized by significant birefringence, with acoustic back-scattering (arrows). (D and E) OCT shows a lesion with clear borders and regions of late enhancement and low signal, identifying a large calcified lesion (arrows).
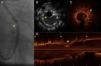
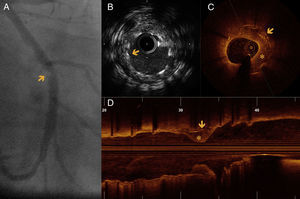
A 37-year-old man presented ST-elevation myocardial infarction in 2010, treated with primary angioplasty to the left circumflex artery (LCx) with implantation of a Taxus DES. One year later, he presented stent restenosis that required implantation of a Cypher DES in the same lesion. Cardiac catheterization was then repeated due to persistent angina; coronary angiography showed severe focal stent restenosis. IVUS assessment was performed but did not detect stent underexpansion or fracture and was unable to identify the site of the lesion (“black hole”). Given the limitations of IVUS, OCT was performed, which confirmed severe eccentric focal restenosis (minimum luminal area 2.9 mm2), but no evidence of strut fracture or underexpansion. The patient had a poorly developed right coronary artery and a new severe proximal LAD lesion, and was therefore referred for coronary artery bypass grafting (Figure 3).
(A) Angiography of the left circumflex artery in right anterior oblique caudal view, showing a focal in-stent lesion with defined edges, difficult to interpret (arrow). (B) IVUS in a region where there are two overlapping stents, with correct apposition, without apparent neointimal proliferation (arrow) and no visible injury to explain the angiographic findings. (C and D) OCT images, in C showing the stent zone overlap (arrow), with significant restenosis (asterisk). Longitudinal OCT reconstruction (D) shows focal restenosis (asterisk) on the overlapping struts (arrows).
Currently, IVUS is the most widely used intracoronary imaging method in routine practice. IVUS images are derived using ultrasound at a frequency of 20–40 MHz, which provides an axial resolution of 100–200 μm and a lateral resolution of around 250 μm. Compared with simple contrast angiography, IVUS can assess stent expansion and apposition after implantation, as well as determine the histology and morphology of atherosclerotic plaques (calcified, fibrous, lipid-rich) and monitor their evolution. IVUS is three times more powerful in detecting calcifications than angiography, with a sensitivity and specificity of 89% and 97%, respectively. These features enable the technique to identify microscopic changes in the size of atheromas and assess their limits and surface, and determine the histological composition of atherosclerotic plaques (calcium, lipids, fibrosis, necrotic core). IVUS can measure the artery wall and its layers, enabling precise selection of stent diameter and length. These properties are useful to visualize deep structures, but limit the study of microstructure, resulting in a sensitivity of only 37% for detection of plaque rupture.1
By contrast, OCT uses reflections of infrared light, providing better spatial resolution and faster data acquisition, and uses smaller diameter catheters than IVUS. OCT's high resolution (better than 15 μm) enables accurate assessment of stent strut positioning and endothelization and determination of the histological components of coronary plaques (microcalcium deposits, macrophage accumulation). However, its low axial penetration (1.5–2 mm) does not provide optimal visualization of the arterial wall, especially in large vessels, in which the artery's outer layers cannot be identified.2 It also requires the injection of contrast during imaging, which means that the main limitation of the technique is that it cannot be performed in situations where there is no coronary flow, such as complete occlusion due to thrombosis or dissection. Furthermore, the need for contrast increases the risk of nephropathy in some patients.2
IVUS and OCT are useful in the study of different coronary diseases. However, the two methods can be used in a complementary way, in order to arrive at a definitive diagnosis and decide on an appropriate invasive strategy.
Calcified lesionsThe presence and extent of calcium in coronary lesions is directly related to the appearance of questionable (“hazy”) images on conventional angiography, especially in eccentric lesions. Calcium shows up as a radiopaque image of lower density than luminal iodine contrast, which makes it very difficult to interpret in certain situations.3 In such cases, intracoronary imaging methods can be useful, considering that angiography has low sensitivity for diagnosing mild, moderate and extensive calcified lesions; IVUS is a valuable tool to identify this type of atheroma.4
Furthermore, OCT can quantify calcium and define its limits. Calcification shows up as areas with low-intensity signal and little back-scattering. However, due to the method's low penetration, only focal calcium plaques not exceeding 1.0–1.5 mm in thickness can be studied.5
Stent restenosisSince OCT has higher definition compared to IVUS, it is better at identifying stent endothelization. Although it is not able to discriminate neointimal proliferation less than 10 μm, OCT can visualize the position of struts and their relationship with the arterial wall, as well as the tissue type of endoluminal endothelization. It can also identify non-uniform strut spacing in different sectors and the presence of microdissections that may cause stent thrombosis. Bouma et al. reported that in images of 42 stents, OCT identified dissections, plaque prolapse and malapposition in fifty percent more cases than with the use of IVUS.6
The different types of stent endothelization are being studied by various research groups. Otake et al. demonstrated that stent struts uncovered by a neointimal layer have a higher incidence of thrombosis.7 However, it is now recognized that not all endothelization processes are the same, but vary according to different stent models. So, in these cases, OCT may have some advantage over IVUS, since the latter does not detect neointima in more than 50% of cases.7
Thrombotic lesionsIn both thrombotic lesions in native arteries and in stent thrombosis, OCT has been shown to be more sensitive than IVUS for identifying the characteristics of the thrombus and possible trigger of the event.8,9 Previous studies highlighted the ability of OCT to differentiate white thrombi and red thrombi, which IVUS cannot do.10 OCT is better at diagnosing these lesions than angiography and IVUS, and it can identify elements that give rise to thrombotic phenomena, such as strut ruptures, stent malapposition and dissections.11
In atherosclerotic plaque ruptures, OCT has the ability to visualize the thin fibrotic cap of the plaque, locate the site of plaque rupture, quantify macrophage accumulation and thus identify the event zone. Similarly, in both acute and late stent thrombosis, OCT reveals zones of inadequate stent endothelization, broken struts or stent malapposition that may be the cause of thrombosis.2,11
In our experience, the importance of establishing the correct diagnosis and lesion characterization using intracoronary imaging lies in providing the interventional cardiologist the necessary information to accurately design the therapeutic strategy. In consequence it increases the chance of success, reducing complications and optimizing long and short-term outcomes.
ConclusionsBoth OCT and IVUS are very useful intravascular imaging methods, each with its advantages and limitations. Although IVUS imaging is the most commonly used method in coronary intervention, OCT has higher resolution, which enables a more detailed assessment of certain coronary lesions. There are as yet no randomized studies attesting the superiority of OCT over IVUS in all cases, but the use of both methods in a complementary manner can be helpful for the correct assessment of coronary disease, enabling the cardiologist to decide the optimal therapeutic strategy to follow.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.