Multi-site pacing is emerging as a new method for improving response to cardiac resynchronization therapy (CRT), but has been little studied, especially in patients with atrial fibrillation. We aimed to assess the effects of triple-site (Tri-V) vs. biventricular (Bi-V) pacing on hemodynamics and QRS duration.
MethodsThis was a prospective observational study of patients with permanent atrial fibrillation and ejection fraction <40% undergoing CRT implantation (n=40). One right ventricular (RV) lead was implanted in the apex and another in the right ventricular outflow tract (RVOT) septal wall. A left ventricular (LV) lead was implanted in a conventional venous epicardial position. Cardiac output (using the FloTrac™ Vigileo™ system), mean QRS and ejection fraction were calculated.
ResultsMean cardiac output was 4.81±0.97 l/min with Tri-V, 4.68±0.94 l/min with RVOT septal and LV pacing, and 4.68±0.94 l/min with RV apical and LV pacing (p<0.001 for Tri-V vs. both BiV). Mean pre-implantation QRS was 170±25 ms, 123±18 ms with Tri-V, 141±25 ms with RVOT septal pacing and LV pacing and 145±19 with RV apical and LV pacing (p<0.001 for Tri-V vs. both BiV and pre-implantation). Mean ejection fraction was significantly higher with Tri-V (30±11%) vs. Bi-V pacing (28±12% with RVOT septal and LV pacing and 28±11 with RV apical and LV pacing) and pre-implantation (25±8%).
ConclusionTri-V pacing produced higher cardiac output and shorter QRS duration than Bi-V pacing. This may have a significant impact on the future of CRT.
O pacing multi-site está a emergir como um novo método de ressincronização cardíaca. Todavia, foi pouco estudado, sobretudo em fibrilhação auricular. Este estudo visa aferir o efeito hemodinâmico e na duração do QRS de pacing Tri-V versus Bi-V.
MétodosEstudo prospetivo observacional de doentes com fibrilhação auricular permanente e fração de ejeção < 40% submetidos a implantação de CRT (n=40). Implantou-se um eletrocateter direito no ápex, outro na parede septal do trato de saída direito e outro em posição venosa epicárdica esquerda convencional. Calcularam-se o débito cardíaco (usando o sistema Vigileo Flotrac®), o QRS médio e a fração de ejeção.
ResultadosO débito cardíaco médio foi 4,81 ± 0,97 L/min em Tri-V, 4,68 ± 0,94 L/min com pacing septal e esquerdo e 4,68 ± 0,94 L/min com pacing apical e esquerdo (p < 0,001 para Tri-V versus ambos BiV). O QRS pré-implantação médio foi 170 ± 25 ms, 123 ± 18 ms em Tri-V, 141 ± 25 ms em pacing septal e esquerdo e145 ± 19 em pacing apical e esquerdo (p < 0,001 para Tri-V versus ambos BiV e pré-implantação). A fração de ejeção média foi estatisticamente superior em Tri-V (30 ± 11%) versus Bi-V (28 ± 12% em pacing septal e esquerdo e 28 ± 11em pacing apical e esquerdo), e versus pré-implantação (25 ± 8%).
ConclusãoO pacing em Tri-V produziu um débito cardíaco superior e QRS mais estreito do que em Bi-V. Estes resultados poderão modificar o futuro da terapêutica de ressincronização.
atrial fibrillation
atrioventricular
biventricular pacing
cardiac resynchronization therapy
electrocardiographic
ejection fraction
left ventricular
New York Heart Association
right ventricular
right ventricular outflow tract
triple-site ventricular pacing
Cardiac resynchronization therapy (CRT) is an established therapy for patients with drug-refractory heart failure and electrical evidence of dyssynchrony. Several large clinical trials have demonstrated that biventricular (Bi-V) pacing significantly reduces all-cause mortality and heart failure-related hospitalizations and symptoms, and improves quality of life, exercise tolerance and left ventricular (LV) systolic performance.1 However, even when appropriately selected, 20-30% of patients do not respond to CRT. This may be due to incomplete resynchronization, as intraventricular and interventricular dyssynchrony can persist in 25-30% of patients despite CRT.2 For such non-responders, multi-site and multi-point pacing are emerging as new methods of CRT. The few previously published studies regarding these novel pacing modalities have shown improved LV hemodynamics and synchrony.3–6 However, doubts remain concerning their indications and short-term and long-term efficacy and safety, and thus the optimal method of resynchronization remains unknown.
In the subgroup of patients with atrial fibrillation (AF), no fewer than 23% of all those with implanted CRT devices,7 studies are still scarce, and these patients are particularly challenging. However, currently available evidence suggests CRT may be of benefit. A meta-analysis8 of prospective cohort studies comparing response to CRT in patients with sinus rhythm vs. AF showed not only that CRT was beneficial in both groups, but also that the AF group performed better in terms of reverse remodeling (assessed as improvement in ejection fraction [EF]), albeit with worse functional outcomes. Because achieving high levels of pacing is harder in patients with AF, there is evidence that atrioventricular (AV) junction ablation may further enhance CRT response and improve not only remodeling response but also functional outcomes.9 Interestingly, there is evidence that even AF patients without severely depressed EF, prolonged QRS or severe heart failure (i.e. in New York Heart Association [NYHA] class ≤II) may benefit from CRT. A randomized trial10 assessing patients who underwent AV junction ablation due to severely symptomatic permanent AF showed that CRT was more beneficial than conventional right ventricular (RV) apical pacing both in patients who met the current recommendations for CRT implantation regarding EF, QRS duration and functional class and in those who did not. Finally, only one trial of multi-site pacing has been undertaken in the subgroup of AF patients,11 with good results.
Thus, to date, the optimal method of cardiac resynchronization remains to be established, especially in patients with AF. We are currently undertaking an observational prospective trial to assess this issue. In this paper we present our results from the acute post-implantation phase, with particular focus on hemodynamic performance.
In this study cardiac resynchronization with multi-site pacing was achieved by means of two right ventricular (RV) leads. Most studies on multi-site pacing used two LV leads and one RV lead.12-14 Only two trials using two RV leads have been published thus far.5,13 Given that the former approach has shown to have greater procedure duration, radiation exposure and LV pacing threshold12,15 and that multi-site pacing using two RV leads has never been assessed in patients with AF, we chose to use the latter approach.
Since the benefit of right ventricular outflow tract (RVOT) septal pacing is still regarded as controversial,14 we also assessed the performance of RVOT septal pacing vs. RV apical pacing as a secondary objective, in order to shed further light on this question.
ObjectiveThe primary aim of this study was to assess the effects of triple-site ventricular (Tri-V) pacing vs. Bi-V pacing on hemodynamic performance and QRS duration. The secondary aim was to assess the effects of RVOT septal pacing vs. RV apical pacing on hemodynamic performance and QRS duration.
MethodsPatient selectionPatients meeting all the following criteria were included: (1) permanent AF; (2) EF <40%; (3) heart failure NYHA ≥II despite appropriate medical treatment and baseline QRS >120 ms or need for anti-bradycardia pacing with an anticipated percentage of ventricular pacing >40%; (4) cognitive capacity to understand the study and hence give informed consent.
Implantation and connection methodWhen a CRT defibrillator device was implanted, the defibrillator lead was positioned in the RV apex, and for CRT pacemaker implantation, one conventional RV lead was implanted in the RV apex. In both cases, a second RV lead was implanted in the RVOT septal wall meeting fluoroscopic criteria14 combined with electrocardiographic (ECG) criteria for correct placement,16 ensuring avoidance of the RVOT anterior or free wall. Finally, a coronary sinus lead for LV pacing was implanted as usual for CRT pacing (i.e. in a posterolateral mid-basal position). The RV apical lead was connected to the RV channel. If the LV lead was bipolar, with a pacing threshold below 2.5 V and no diaphragmatic stimulation, the LV lead was connected to the atrial channel and the RVOT lead connected to the LV channel. If the LV lead was quadripolar, if the pacing threshold was over 2.5 V, if specific LV vector programming was necessary, or if there was any type of diaphragmatic stimulation, then the LV lead was connected to the LV channel and the RVOT lead was connected to the atrial channel on the generator. Any device and lead brand could be used. Programming was set to DDDR with the lowest possible AV interval (25-40 ms depending on device brand) and a VV interval of 0 ms.
Hemodynamic assessmentUp to one month after implantation, all patients underwent minimally invasive hemodynamic assessment with placement of a radial arterial line using the FloTrac III™ Vigileo™ monitoring system (Edwards Lifesciences, Irvine, CA, USA), which calculates cardiac output based on the arterial pressure waveform. This system is widely used in peri- and intraoperative settings as well as in intensive care units, and is useful in detecting sudden changes in cardiac output and volume status provided the cardiac rhythm is regular.17
Cardiac output and systolic volume were assessed at 70 beats per minute in the following configurations: RV apical, RV RVOT, LV, Bi-V pacing using the RV apical (RVA) and LV leads (Bi-V RVA-LV), Bi-V pacing using the RVOT and LV leads (Bi-V RVOT-LV), and Tri-V. The assessment was performed under ≥99% pacing. In order to achieve these levels, AV node suppression was achieved previously by means of pharmacotherapy or by AV node ablation in drug-refractory cases. Each configuration was assessed for 15 min to achieve stable measurements. During transitions, to avoid a potential carry-over effect, the first 5 min of measurements were disregarded, and only measurements from the following 15 min were considered. The operator performing the hemodynamic assessment was blinded to the pacing mode used.
Electrocardiographic assessmentSimultaneously with the hemodynamic assessment, all patients underwent standard 12-lead ECG assessment in each of the following configurations: RV apical, RV RVOT, LV, Bi-V RVA-LV, Bi-V RVOT-LV and Tri-V. QRS duration was measured in each of these configurations by two blinded operators.
Echocardiographic assessmentThe echocardiographic study was performed with a Vivid 7 device (General Electric®) in each of the following configurations: Tri-V, Bi-V RVA-LV and Bi-V RVOT-LV. The study was undertaken simultaneously with the hemodynamic assessment.
EF was estimated by the Simpson biplane method determined from the cine loops acquired in two-dimensional mode, end-diastolic and late systolic volumes being measured in three consecutive cardiac cycles. Post-processing analysis was performed by two operators using EchoPAC© software (General Electric).
Statistical analysisMean cardiac output, QRS and EF were calculated in each group. Differences were assessed by a paired samples t test.
In order to assess differences in procedure duration, fluoroscopy duration and radiation dosage, the additional amounts of these parameters required for implantation of the second RV lead were quantified, and the differences between Bi-V and Tri-V were assessed by the t test.
Ethical considerationsThis paper conforms with the Helsinki Declaration on the Ethical Principles for Medical Research Involving Human Subjects and was approved by our local ethics committee. Written informed consent was obtained from all patients.
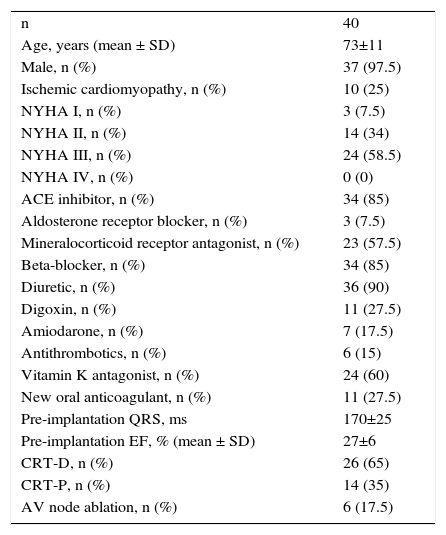
ResultsPopulationA total of 40 patients were included. Baseline pre-implantation characteristics are summarized in Table 1. Most patients were severely symptomatic, with markedly depressed EF and a significantly prolonged QRS. AV node ablation was necessary in six patients prior to hemodynamic assessment.
Patient population details.
| n | 40 |
| Age, years (mean ± SD) | 73±11 |
| Male, n (%) | 37 (97.5) |
| Ischemic cardiomyopathy, n (%) | 10 (25) |
| NYHA I, n (%) | 3 (7.5) |
| NYHA II, n (%) | 14 (34) |
| NYHA III, n (%) | 24 (58.5) |
| NYHA IV, n (%) | 0 (0) |
| ACE inhibitor, n (%) | 34 (85) |
| Aldosterone receptor blocker, n (%) | 3 (7.5) |
| Mineralocorticoid receptor antagonist, n (%) | 23 (57.5) |
| Beta-blocker, n (%) | 34 (85) |
| Diuretic, n (%) | 36 (90) |
| Digoxin, n (%) | 11 (27.5) |
| Amiodarone, n (%) | 7 (17.5) |
| Antithrombotics, n (%) | 6 (15) |
| Vitamin K antagonist, n (%) | 24 (60) |
| New oral anticoagulant, n (%) | 11 (27.5) |
| Pre-implantation QRS, ms | 170±25 |
| Pre-implantation EF, % (mean ± SD) | 27±6 |
| CRT-D, n (%) | 26 (65) |
| CRT-P, n (%) | 14 (35) |
| AV node ablation, n (%) | 6 (17.5) |
ACE: angiotensin-converting enzyme; CRT-D: cardiac resynchronization therapy defibrillator; CRT-P: cardiac resynchronization therapy pacemaker; EF: ejection fraction; NYHA: New York Heart Association; SD: standard deviation.
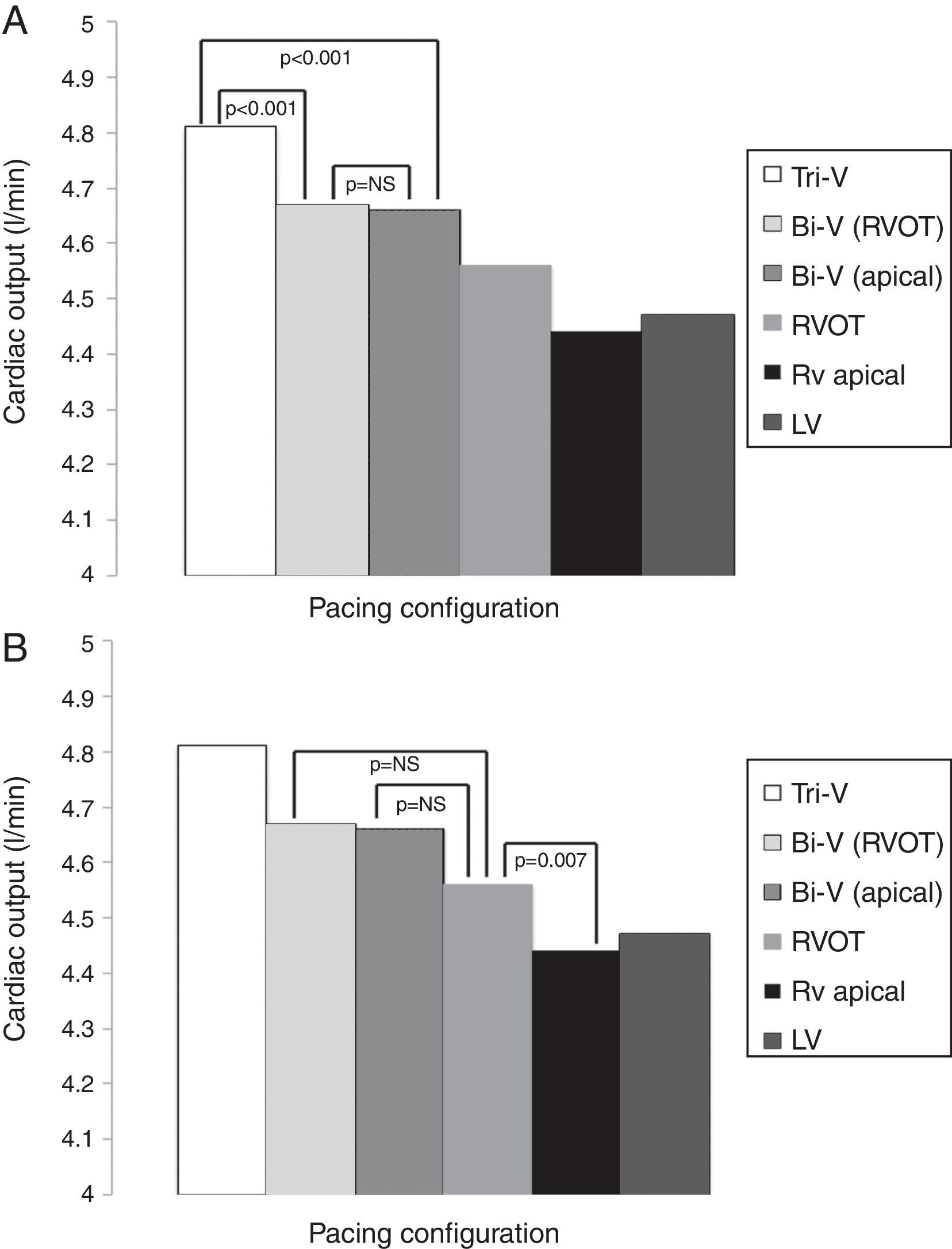
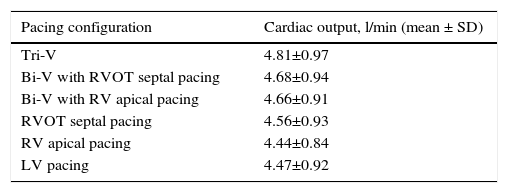
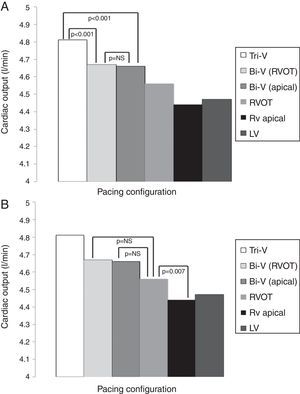
The hemodynamic results are depicted in Table 2 and Figures 1A and B. Cardiac output was significantly higher during Tri-V compared to Bi-V pacing. There was no statistically significant difference between the two Bi-V pacing configurations, nor between Bi-V pacing and RVOT septal pacing. The latter produced a significantly higher cardiac output than RV apical pacing. These differences remain across ischemic and non-ischemic cardiomyopathy groups.
Cardiac output in different pacing configurations.
| Pacing configuration | Cardiac output, l/min (mean ± SD) |
|---|---|
| Tri-V | 4.81±0.97 |
| Bi-V with RVOT septal pacing | 4.68±0.94 |
| Bi-V with RV apical pacing | 4.66±0.91 |
| RVOT septal pacing | 4.56±0.93 |
| RV apical pacing | 4.44±0.84 |
| LV pacing | 4.47±0.92 |
Bi-V: biventricular pacing; LV: left ventricular; RV: right ventricular; RVOT: right ventricular outflow tract; Tri-V: triple-site pacing.
Differences in cardiac output between Tri-V and Bi-V pacing (A), between Bi-V pacing and RVOT septal pacing, and between RVOT septal pacing and RV apical pacing (B). Bi-V: biventricular pacing; LV: left ventricular; RV: right ventricular; RVOT: right ventricular outflow tract; Tri-V: triple-site pacing.
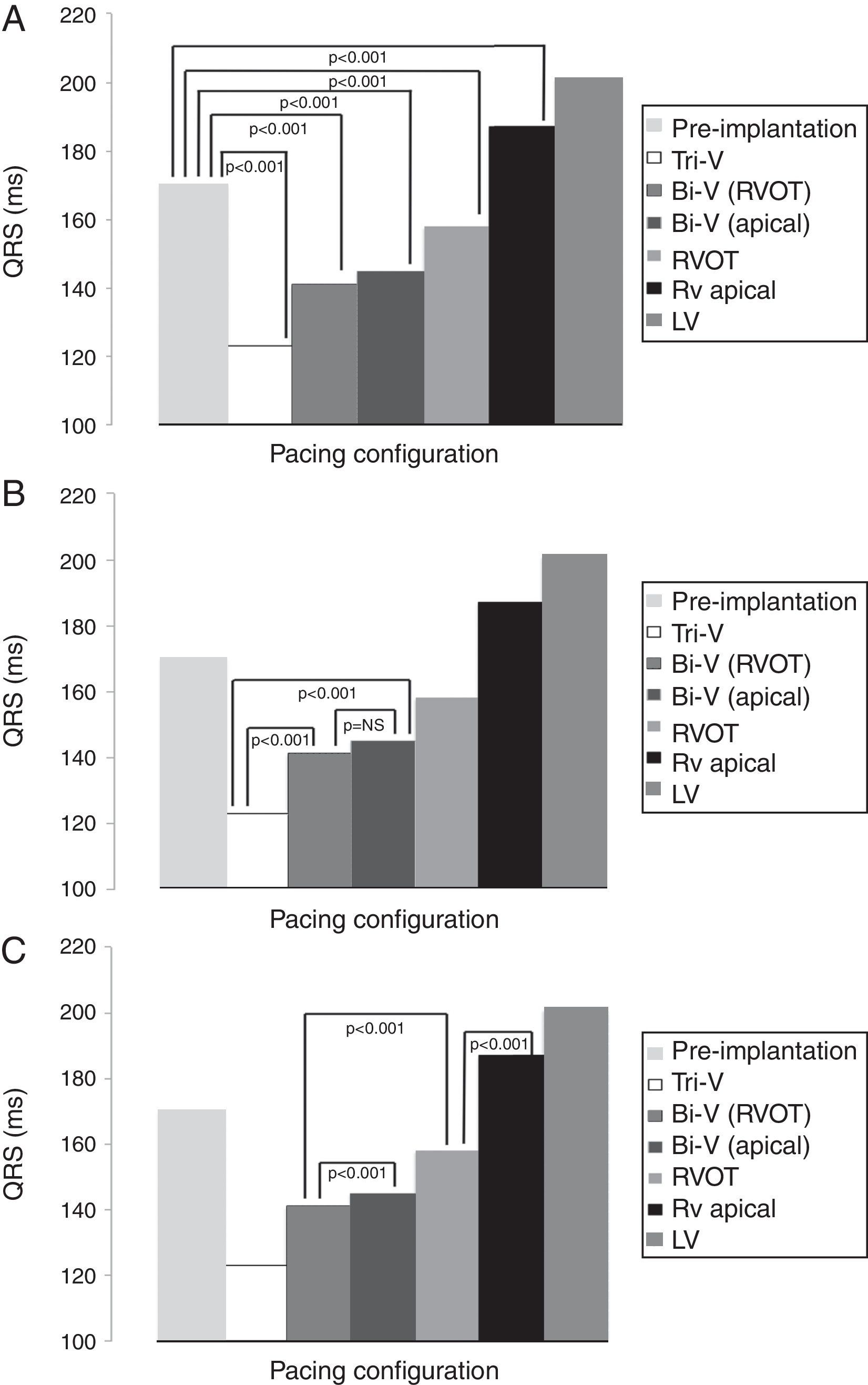
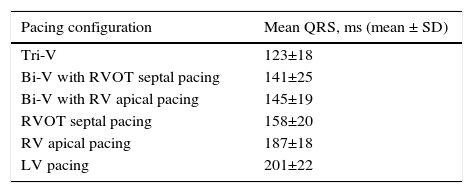
Mean QRS duration in each pacing configuration is depicted in Table 3 and Figures 2A, B and C. Tri-V pacing produced a significantly shorter QRS than pre-implantation QRS and both Bi-V pacing configurations. Bi-V pacing also significantly shortened QRS duration compared with pre-implantation QRS. There was no statistically significant difference between the two Bi-V pacing configurations. RVOT septal pacing produced a significantly shorter QRS than pre-implantation QRS and RV apical pacing QRS, but a significantly longer QRS than Bi-V pacing. These differences remain across ischemic and non-ischemic cardiomyopathy groups.
QRS duration in each pacing configuration.
| Pacing configuration | Mean QRS, ms (mean ± SD) |
|---|---|
| Tri-V | 123±18 |
| Bi-V with RVOT septal pacing | 141±25 |
| Bi-V with RV apical pacing | 145±19 |
| RVOT septal pacing | 158±20 |
| RV apical pacing | 187±18 |
| LV pacing | 201±22 |
Bi-V: biventricular pacing; LV: left ventricular; RV: right ventricular; RVOT: right ventricular outflow tract; Tri-V: triple-site pacing.
Differences in mean QRS between pre-implantation and pacing (A), between Tri-V and Bi-V pacing (B), between Bi-V and RVOT septal pacing, and between the latter and RV apical pacing (C). Bi-V: biventricular pacing; LV: left ventricular; RV: right ventricular; RVOT: right ventricular outflow tract; Tri-V: triple-site pacing.
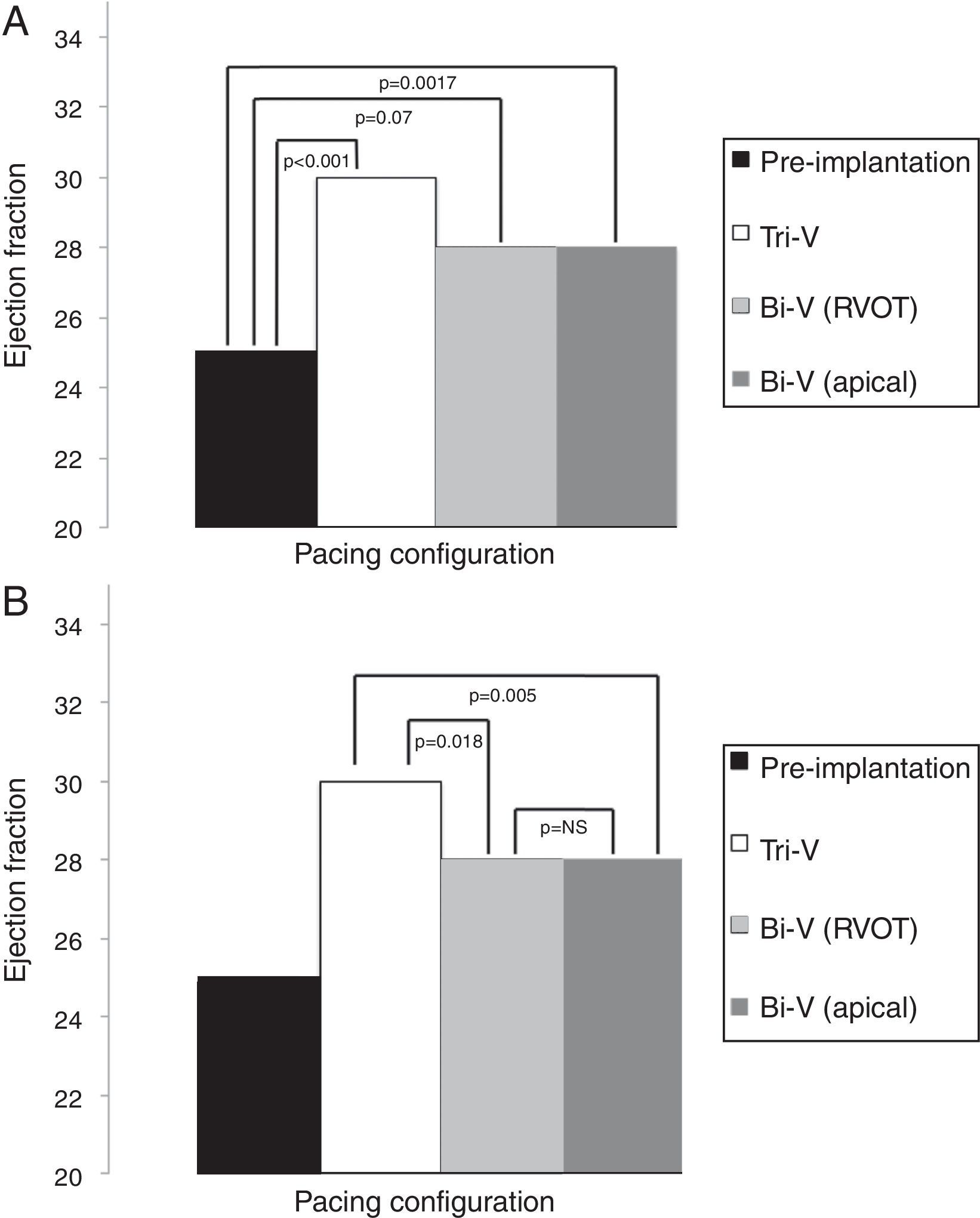
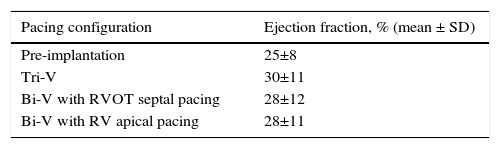
EF results are depicted in Table 4 and Figures 3A and B. All modes of CRT produced a statistically significant improvement in EF. Tri-V produced the greatest improvement, with a significant difference compared to Bi-V. These differences remain across ischemic and non-ischemic cardiomyopathy groups.
Echocardiographic ejection fraction in each pacing configuration.
| Pacing configuration | Ejection fraction, % (mean ± SD) |
|---|---|
| Pre-implantation | 25±8 |
| Tri-V | 30±11 |
| Bi-V with RVOT septal pacing | 28±12 |
| Bi-V with RV apical pacing | 28±11 |
Bi-V: biventricular pacing; RV: right ventricle; RVOT: right ventricular outflow tract; Tri-V: triple-site pacing.
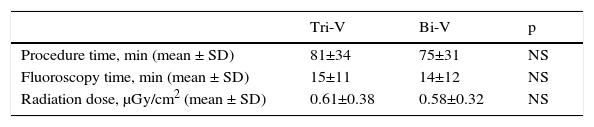
Although implantation of the Tri-V system resulted in longer procedure and fluoroscopy times, the difference was not significant compared to conventional Bi-V implantation (Table 5).
Procedure and fluoroscopy times and radiation dose in Tri-V pacing vs. conventional Bi-V pacing.
| Tri-V | Bi-V | p | |
|---|---|---|---|
| Procedure time, min (mean ± SD) | 81±34 | 75±31 | NS |
| Fluoroscopy time, min (mean ± SD) | 15±11 | 14±12 | NS |
| Radiation dose, μGy/cm2 (mean ± SD) | 0.61±0.38 | 0.58±0.32 | NS |
Bi-V: biventricular pacing; Tri-V: triple-site pacing.
In this study, Tri-V pacing produced a superior hemodynamic performance compared to conventional Bi-V pacing, as demonstrated by increased cardiac output and EF. This is likely an immediate consequence of improved resynchronization, given that QRS duration was significantly shorter in Tri-V pacing vs. conventional Bi-V pacing, and that this was an acute phase study, in which reverse remodeling could not yet have occurred. The reduction in QRS duration obtained by Tri-V pacing is especially remarkable, QRS having been shortened almost to normal length. Our fluoroscopy and procedure times were scarcely prolonged by this technique, and as such were not a significant limitation.
There have been few studies on multi-site pacing, and the methodology has been very heterogeneous regarding not only pacing and implant techniques, but also the method used for assessing hemodynamic response. Most used two LV leads and one RV lead. In one non-randomized trial one of the LV leads was connected to the atrial channel (all patients had permanent AF), and the other two leads were connected as usual. Triple-site pacing yielded higher EF and reduced end-systolic volume, without clinical improvement, at three months.11 Of course, by connecting an LV lead to an atrial channel, the use of vectors is limited. Two other randomized trials used a similar approach but connected both LV leads with a Y-connector. They demonstrated an additional clinical benefit, but greater procedure length, radiation exposure and LV pacing threshold.12,15 Regarding safety, none of these trials have shown significant differences between Tri-V or Bi-V pacing, and Ogano et al.18 recently demonstrated that dual LV pacing might even have a protective effect against ventricular arrhythmias.3
Only three trials using two RV leads and one LV lead alone have been published.5,13,19 However, in two of these, the connection technique was different, as both RV leads were connected to the atrial channel by means of a Y-connector. Systolic function was assessed invasively by determining peak LV dP/dt (using a micromanometer-tipped pigtail angiographic catheter) and also cardiac output with a Swan-Ganz catheter. Dyssynchrony was assessed with tissue Doppler echocardiographic imaging. QRS duration was also assessed in each pacing configuration. Triple-site pacing was superior regarding all four parameters compared to conventional BiV pacing. There were also no safety issues regarding this method. Finally, one trial used both types of triple-site pacing (i.e. one group received two RV leads, and another two LV leads), with better clinical and hemodynamic results in triple-site pacing compared to conventional Bi-V pacing.20 A very recent randomized trial compared Tri-V pacing with conventional Bi-V pacing in terms of clinical and echocardiographic data at 12-month follow-up. There were no differences in adverse events or clinical benefit, however Tri-V did produce higher EF at 12 months.5,13,19
Despite the heterogeneity of the studies, and the unique methodology of our study, all of these trials using triple-site pacing have demonstrated superior hemodynamic performance and increased resynchronization compared to conventional Bi-V pacing. The benefit was also present in AF patients.11 Thus, our results are consistent with previous studies.
It is worth noting that no previous trial has ever assessed the use of triple-site pacing with two RV leads in patients with permanent AF. Given that these patients represent an understudied and particularly challenging group, our study adds pioneering scientific data on this modality of cardiac resynchronization therapy in this subgroup.
Regarding the results of single-site pacing, our study produced interesting results. RVOT septal pacing was clearly superior to RV apical pacing, with improved cardiac output and much shorter QRS duration. RVOT septal pacing alone even decreased baseline QRS length in our patients. Studies comparing these two pacing modalities have produced conflicting results.14 However, the discrepancies are largely due to the significant heterogeneity in lead placement technique: few studies have been careful enough to ensure proper lead placement in both the RVOT and the septal wall. This was a critical concern in our study. Several trials in which the RV lead was placed under careful fluoroscopic and/or electrocardiographic guidance yielded superior results for RVOT septal pacing.14 Our study adds further weight to this evidence.
Finally, the absence of a statistical difference between cardiac output with Bi-V pacing and RVOT septal pacing was an unexpected finding, especially considering that the QRS was significantly shorter with Bi-V pacing. The clinical significance of this finding is unclear, given that we were not comparing the clinical performance of these two pacing modalities. Studies comparing single-site RV pacing vs. Bi-V pacing used echocardiographic and clinical endpoints obtained some time after implantation, and not immediate hemodynamic data.21 In addition, the majority of these studies compare RV apical pacing rather than RVOT septal pacing. Thus, no study can be directly compared to ours, and while most studies favor Bi-V pacing, the above-mentioned limitations regarding RV pacing site may be of greater importance than previously thought. Therefore, whatever the interpretation of our particular results, they highlight the importance and potential of RVOT septal pacing. Indeed, in patients in whom the CRT indication is based on need for pacing rather than primarily on heart failure, could RVOT septal pacing be a sustainable alternative to Bi-V pacing? Our trial was neither designed nor statistically powered to answer such a question, but adds further evidence to the issue.
LimitationsOur study has several limitations. Use of the FloTrac III™ Vigileo™ monitoring system is time-consuming and while it has been well validated in a variety of clinical scenarios,17 this is the first time that it has been tested in this setting. Also, ischemic cardiomyopathy was found in only 25% of all patients. Despite the fact that statistical results were similar across this subgroup of patients, the absolute number of patients is small, and as such this heterogeneity may have influenced our results. Finally, although the echocardiographic results were statistically significant, the resulting differences are small and within the inter- and intraobserver variability of the technique. Thus, these particular results should be interpreted with caution. The observed differences in cardiac output were also small, and therefore we cannot yet be absolutely sure of their clinical significance.
Future directionsThe fact that multiple techniques, including ours, yielded benefit suggests that multi-site pacing should be further developed. The optimal method, whether with two RV leads, two LV leads, a single lead with multi-point pacing capabilities or a combination of these remains to be determined. We believe that if three ventricular leads are to be used, two RV leads are much faster and probably more stable. The development of CRT generators designed specifically for multi-site pacing may also be important. So far only one manufacturer has produced such a device, which will be tested in non-responders in a randomized trial.22 It is worth pointing out, however, that in our population we deliberately did not select a subset of non-responders, and still achieved superior results with this new pacing modality. This raises the possibility of organizing a large trial of multi-site pacing as a first-line treatment in candidates for cardiac resynchronization therapy.
ConclusionTriple-site pacing produced a superior hemodynamic performance with shorter QRS duration, which is likely the consequence of improved resynchronization. RVOT septal pacing was clearly superior to RV apical pacing, with shorter QRS and greater cardiac output.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingNo specific funding grants were used regarding this study.
Conflicts of interestThe authors have no conflicts of interest to declare.


 Tri-V and
Tri-V and 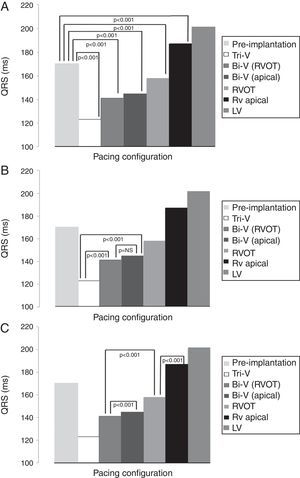 Tri-V and
Tri-V and 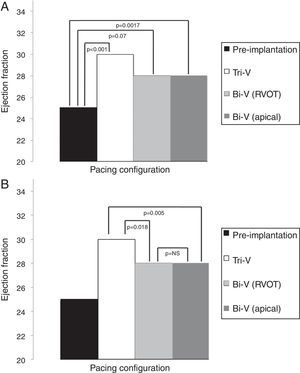 Tri-V and
Tri-V and 

