The benefits of new technologies almost always come at a cost, often creating new problems, for which new solutions must be sought. The development of coronary stents is a perfect example of this.
The first bare-metal stents (BMS) revolutionized percutaneous coronary intervention, since they overcame the acute and late elastic recoil seen after balloon angioplasty, thus significantly reducing the risk of restenosis, and solved the problem of acute vessel closure associated with balloon angioplasty due to vessel dissection.1 However, despite these benefits, BMS were associated with neointimal hyperplasia due to deep arterial injury, resulting in in-stent restenosis in 15-30% of cases.2 Stents eluting antiproliferative agents were the next step. The first generation of drug-eluting stents (DES) used sirolimus (Cypher, Cordis J&J) and paclitaxel (Taxus, Boston Scientific), incorporated in biostable polymers coating stainless steel stent platforms. These stents showed a significant reduction in in-stent restenosis, late lumen loss and rate of target lesion/vessel revascularization compared with BMS.3 However, possibly due to delayed endothelialization secondary to antiproliferative drug elution and also hypersensitivity reactions to the polymer coating, these stents were later associated with an increased risk of death and myocardial infarction, due to late and very late stent thrombosis.4
In order to deal with this problem, new polymers with more biocompatible molecules (such as zotarolimus and everolimus) were developed, enabling faster drug elution, and providing earlier endothelial coverage. These polymers were applied in new metal alloys, like cobalt-chromium, which enabled reductions in strut thickness and a lower risk of allergic reactions. These second-generation DES included, among others, Xience (Abbott), Endeavor (Medtronic) and Promus (Boston Scientific). Overall, they were shown to significantly reduce rates of myocardial infarction, target lesion revascularization and stent thrombosis, compared with first-generation DES.3
Although the long-term safety of these stents was better than that reported for BMS or first-generation DES, concerns persisted about the risk of late and very late stent thrombosis and the need for prolonged dual antiplatelet therapy. This risk has been associated with the durable polymer coating of DES, which may promote hypersensitivity reactions in the coronary artery after stent deployment.5 This hypothetical pathophysiological mechanism led to the development of new DES platforms, using biodegradable instead of durable polymers. Several stents of this new third generation of DES have been developed over recent years. Besides using biodegradable polymers, most of these new stents also use cobalt-chromium or platinum-chromium platforms (allowing ultra-thin struts) and several have only abluminal polymer distribution (Table 1).
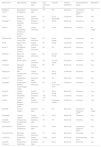
Biodegradable polymer stents and drug-eluting stents without a polymer.
| Stent name | Manufacturer | Platform alloy | Strut thickness, μm | Polymer | Polymer distribution | Anti-proliferative agent | Marketed? |
|---|---|---|---|---|---|---|---|
| BioMatrix Flex™ | Biosensors (Singapore) | Stainless steel | 112 | PA | Abluminal | Umirolimus (Biolimus-A9TM) | Yes |
| Orsiro | Biotronik (Germany) | Cobalt-chromium | 61 | PLLA (BIOlute®) | Conformal | Sirolimus | Yes |
| Synergy™ | Boston Scientific (USA) | Platinum-chromium | 74 | PLGA | Abluminal | Everolimus | Yes |
| Nevo™ | Cordis Corporation, Johnson & Johnson (USA) | Cobalt-chromium | 99 | PLGA | Reservoirs | Sirolimus | No longer |
| DESyne BD | Elixir Medical Corp (USA) | Cobalt-chromium | 81 | PLLA-based | Conformal | Novolimus | Yes |
| Tivoli | Essen Tech (China) | Cobalt-chromium | 80 | PLGA | Conformal | Sirolimus | No |
| Excel II | JW Medical System (China) | Cobalt-chromium | 88 | PA | Abluminal | Sirolimus | Yes |
| BioMime | Meril Life Sciences (India) | Cobalt-chromium | 65 | PLLA & PLGA | Conformal | Sirolimus | Yes |
| MiStent | Micell (USA) | Cobalt-chromium | 59 | PLLA & PLGA | Conformal | Sirolimus | Yes |
| Firehawk | Microport Medical (China) | Cobalt-chromium | 86 | PA | Abluminal | Sirolimus | Yes |
| GenXSync | MIV Therapeutics (India) | Cobalt-chromium | 65 | PA & PLGA | Conformal | Sirolimus | Yes |
| Combo™ | OrbusNeich (China) | Stainless steel | 100 | PA | Abluminal | Sirolimus | Yes |
| Supraflex | Sahajanand Medical Technologies (India) | Cobalt-chromium | 60 | PLLA, PLCL and PVP | Conformal | Sirolimus | Yes |
| Inspiron | Scitech (Brazil) | Cobalt-chromium | 75 | PA & PLGA | Abluminal | Sirolimus | Yes |
| BuMA™ | SINOMED (China) | Stainless steel | 100-110 | PLGA | Conformal | Sirolimus | Yes |
| Svelte | Svelte Medical Systems (USA) | Cobalt-chromium | 81 | Poly(ester amide) | Conformal | Sirolimus | Yes |
| Nobori® | Terumo (Japan) | Stainless steel | 81 | PA | Abluminal | Umirolimus (Biolimus-A9TM) | No longer |
| Ultimaster | Terumo (Japan) | Cobalt-chromium | 80 | PLCL | Abluminal | Sirolimus | Yes |
| Yukon® Choice PC | Translumina Therapeutics (India) | Stainless steel | 79 | PA | Abluminal | Sirolimus | Yes |
| BioFreedom™ | Biosensors (Singapore) | Stainless steel | 112 | None | Abluminal | Umirolimus (Biolimus-A9TM) | Yes |
| Amazonia Pax | Minvasys (France) | Cobalt-chromium | 73 | None | Abluminal | Paclitaxel | Yes |
| Cre8 EVO | Alvimedica (Italy) | Cobalt-chromium | 70-80 | None | Abluminal | Sirolimus+fatty acid | Yes |
| Yukon® Choice 4 | Translumina (Germany) | Stainless steel | 87 | None | Abluminal | Sirolimus | Yes |
PA: polylactic acid; PLGA: poly-l-lactide-co-glycolide; PLLA: poly-L-lactide; PLCL: poly DL-lactide-co-caprolactone; PVP: polyvinyl pyrrolidone.
In this issue of the Journal, Prado Jr and colleagues present long-term (five-year) results of one of these stents – the Inspiron™ sirolimus-eluting stent – in comparison with the control Biomatrix™ Flex biolimus-eluting stent.6 The Inspiron stent is an ultra-thin strut (75 μm) cobalt-chromium sirolimus-eluting stent, using a biodegradable polymer (polylactic acid and poly-l-lactide-co-glycolide, with abluminal distribution). Nine-month intracoronary imaging results and one-year angiographic and clinical results have previously been reported, confirming non-inferiority compared to the control Biomatrix™ stent.7,8
Prado Jr et al.’s paper reports clinical non-inferiority of the Inspiron stent, with a similar rate of major adverse events (MACE) in comparison with the control Biomatrix™ stent (12.5% vs. 17.9%, p=0.4) at five-year follow-up. However, importantly, the current trial (like all first-in-man and pilot trials with new stents) was not powered for clinical events. As acknowledged by the authors, the population was small, low risk (mostly stable patients) and included predominantly non-complex lesions (data on lesions is not provided in the current paper, but was reported in a previous publication7). As such, the reported incidence of MACE at five years with the Inspiron stent should be interpreted with caution. Just for perspective, in an all-comers population study including 1707 patients, the Biomatrix™ stent had a five-year MACE rate of 22.3%.9
So, overall, how does this new generation of DES compare with previous ones? In fact, comparisons are difficult, since, besides the biodegradable polymers, these stents also have different strut thickness (thinner overall) and new cell designs.
When compared with the first generation of DES (Cypher), ultra-thin stents with biodegradable polymers reduced risk of target lesion revascularization (hazard ratio [HR] 0.82, 95% confidence interval [CI] 0.68-0.98, p<0.029), risk of stent thrombosis (HR 0.56, 95% CI 0.35-0.90, p<0.015), particularly very late stent thrombosis (HR 0.22, 95% CI 0.08-0.61, p<0.004) and incidence of myocardial infarction (HR 0.59, 95% CI 0.73-0.95, p<0.031) in a pooled analysis at four years of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials, which used the third-generation Yukon Choice and BioMatrix™ Flex stents.10
With regard to comparisons with second-generation DES, trials were largely designed as non-inferiority studies and the most important trials used the Xience stent as a comparator. Overall, the results of these trials were positive (i.e. non-inferiority was met for the third-generation DES). A recent meta-analysis, including nine trials enrolling 10 699 patients with a mean follow-up of 63 months, confirmed individual study results, showing no significant difference in target lesion failure (odds ratio [OR] 1.04, 95% CI 0.89-1.21) and definite/probable stent thrombosis (OR 0.78, 95% CI 0.59-1.01).11
However, two recent studies with the Orsiro stent (an ultra-thin strut cobalt-chromium metallic stent platform releasing sirolimus from a biodegradable polymer) suggested better results. In the first study, a sub-analysis of the BIO-RESORT in small vessels (<2.5 mm), the Orsiro showed significantly lower target lesion revascularization at three years compared to the Xience stent, but similar results to another third-generation stent (Synergy).12 In the second study (BIOSTEMI), the Orsiro stent was superior to the Xience Expedition stent with respect to target lesion failure at one year, mainly due to a reduction in ischemia-driven target lesion revascularization.13
In conclusion, while several other randomized trials and registries with these third-generation stents are recruiting patients all over the world, the available evidence is already reassuring for these devices. Not a bad place to be, particularly when the predicted next step in stent development (absorbable scaffolds) is currently on hold.
Conflicts of interestDr. Baptista has received consultancy and speaker fees from Abbott and Boston Scientific and research grants from Abbott.