The Stents Coated With the Biodegradable Polymer on Their Abluminal Faces and Elution of Sirolimus Versus Biolimus Elution for the Treatment of de Novo Coronary Lesions – DESTINY Trial is a non-inferiority randomized study that compared the Inspiron™ sirolimus-eluting stent (SES) with the control Biomatrix™ Flex biolimus-eluting stent (BES). Previous reports in the first year showed similar outcomes for both stents, in clinical, angiographic, optical coherence tomography, and intravascular ultrasound assessments. The present analysis aims to compare the clinical performance of these two biodegradable polymer drug-eluting stents five years after the index procedure.
MethodsA total of 170 patients (194 lesions) were randomized in a 2:1 ratio for treatment with SES or BES, respectively. The primary endpoint for the present study was the five-year rate of combined major adverse cardiac events, defined as cardiac death, myocardial infarction, or target lesion revascularization.
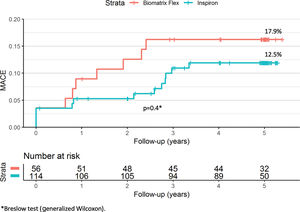
ResultsAt five years, the primary endpoint occurred in 12.5% and 17.9% of the SES and BES groups, respectively (p=0.4). There was no definite or probable stent thrombosis among patients treated with the novel SES stent during the five years of follow-up, and no stent thrombosis after the first year in the BES group.
ConclusionsThe novel Inspiron™ stent had similar good clinical performance in long-term follow-up when compared head-to-head with the control latest-generation Biomatrix™ Flex biolimus-eluting stent.
Stents Coated with the Biodegradable Polymer on their Abluminal Faces and Elution of Sirolimus Versus Biolimus Elution for the Treatment of de Novo Coronary Lesions (DestinyTrial) é um estudo randomizado de não inferioridade que comparou o stent farmacológico eluído com Sirolimus Inspiron® (SES) ao controle o stent Biomatrix® Flex eluído com biolimus (BES). Relatórios dentro do primeiro ano mostraram resultados semelhantes para ambos os stents, em seguimento clínico, angiográfico e também em análise de tomografia de coerência ótica e ultrassom intracoronário. A presente análise tem como objetivo comparar o desempenho clínico desses dois stents farmacológicos com polímeros biodegradáveis após cinco anos do procedimento índice.
MétodosForam randomizados 170 pacientes (194 lesões) em uma proporção de 2:1 para tratamento com SES ou BES, respetivamente. O desfecho primário para o presente estudo foi a taxa em cinco anos de eventos cardíacos adversos maiores combinados, definida como morte cardíaca, infarto do miocárdio ou revascularização da lesão-alvo.
ResultadosEm cinco anos, o desfecho primário ocorreu em 12,5% e 17,9% para o grupo SES e BES, respectivamente (p=0,4). Não houve trombose de stent definitiva ou provável entre os pacientes tratados com o novo SES durante os cinco anos de seguimento e ausência de trombose de stent após o primeiro ano no grupo BES.
ConclusõesO novo stent Inspiron® apresentou uma boa e semelhante performance clínica no seguimento em longo prazo, quando comparado com o controle o stent de última geração Biomatrix® Flex.
Drug-eluting stents (DES) significantly reduce restenosis and the need for repeat intervention compared to bare-metal stents.1 However, over time, concerns have been raised related to their procedural performance and their long-term safety.2 The so-called new-generation DES comprise a heterogeneous group of devices, each incorporating different improvements in one or more features of DES construction. Overall, new-generation DES have been shown to be associated with better outcomes than earlier DES formulations.3 However, new DES do not present a class-effect performance, with previous reports demonstrating that subtle, though sizable, contrasts might exist between different DES.4
Coronary artery disease is a chronic condition, for which any optimal therapeutic strategy must provide a long-lasting protective effect, ideally lifelong. Recent findings have shown that percutaneous coronary intervention with bioabsorbable scaffolds may be associated with ominous complications years after the initial procedure,5 a reminder to the medical community of the importance of keeping track of the very long-term performance of any coronary treatment, including metal DES.
The Stents Coated With the Biodegradable Polymer on Their Abluminal Faces and Elution of Sirolimus Versus Biolimus Elution for the Treatment of de Novo Coronary Lesions – DESTINY Trial is a randomized study that compared the novel Inspiron™ sirolimus-eluting stent (SES) head-to-head with the control Biomatrix™ Flex biolimus-eluting stent (BES).6,7 Previous reports from DESTINY showed similar outcomes in the first year for both stents, in clinical, angiographic, optical coherence tomography, and intravascular ultrasound assessments.6,7
The present analysis aims to compare the long-term clinical performance of the two latest-generation DES five years after the index procedure.
MethodsDetails of the study protocol and the baseline characteristics of the study population, as well as clinical, angiographic, and intravascular imaging results after the first months, have been published elsewhere.6,7 Briefly, DESTINY was initially designed as a non-inferiority trial to compare SES with BES for the primary endpoint of angiographic late lumen loss at nine months.7 Thereafter, patients were followed clinically for five years. Between June and December 2013, patients with one or two de novo lesions (n=170) were included and randomized in a 2:1 ratio for treatment with the Inspiron™ sirolimus-eluting stent (Scitech, Aparecida de Goiania, Brazil) or the Biomatrix™ biolimus-eluting stent (Biosensors Europe SA, Morges, Switzerland), respectively. Dual antiplatelet therapy was recommended for 12 months, followed by lifelong aspirin. Written informed consent was obtained from all patients in accordance with the Declaration of Helsinki, and the local and national ethics committees approved the trial.
In the present study, we report the five-year outcomes of patients included in the DESTINY trial, who were assessed for the occurrence of the primary endpoint of major adverse cardiac events (MACE), defined as cardiac death, myocardial infarction (MI), or target lesion revascularization. Deaths were considered to be cardiac unless unequivocally related to a noncardiac cause. MI was diagnosed as previously proposed.8 Target lesion revascularization was defined as any coronary reintervention (surgical or percutaneous) to treat a lesion located within the stent and its 5-mm proximal and 5-mm distal edges. Stent thromboses were classified according to the definitions proposed by the Academic Research Consortium.9 An independent data safety and monitoring board periodically reviewed the accumulated study data for recommendations on participant safety and efficacy, study conduct, and continuation or modifications. All complications were adjudicated by an independent adverse events committee.
Categorical variables were presented as percentages and compared using Fisher's exact test or the chi-square test. Continuous variables were presented as means and standard deviations and compared using the Student's t test. The risk of adverse events was estimated using the Kaplan-Meier method and compared using the Breslow test (generalized Wilcoxon). A p-value of <0.05 was considered to be significant. Statistical analyses were performed using IBM SPSS version 21.0 (IBM Corporation).
ResultsOverall, 170 patients with 194 treated lesions were included in the study and comprise the final population. Baseline clinical and procedural characteristics of the two groups were similar (Table 1).
Baseline and procedural characteristics of the study population.
| SES (n=114 patients; 132 lesions) | BES (n=56 patients; 62 lesions) | p | |
|---|---|---|---|
| Age, years | 59.9±9.4 | 59.9±9.8 | >0.9 |
| Male | 66 (57.9) | 27 (48.2) | 0.2 |
| Diabetes | 41 (36.6) | 20 (36.4) | >0.9 |
| Prior MI | 51 (45.1) | 23 (41.1) | 0.6 |
| Stable coronary disease | 85 (74.6) | 39 (69.6) | 0.5 |
| Target vessela | |||
| LAD | 60 (45.5) | 27 (43.6) | 0.8 |
| LCx | 34 (25.8) | 18 (29.0) | 0.6 |
| RCA | 38 (28.8) | 17 (27.4) | 0.8 |
| Lesion length, mm | 14.6±6.4 | 15.5±6.8 | 0.4 |
| Reference diameter, mm | 2.74±0.44 | 2.83±0.43 | 0.2 |
| Baseline minimum lumen diameter, mm | 0.89±0.35 | 0.92±0.40 | 0.6 |
| Baseline diameter stenosis, % | 67.6±11.9 | 67.5±12.5 | 0.9 |
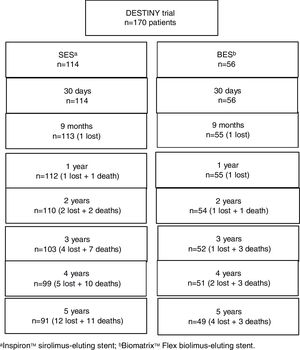
A total of 16 patients (9.4%) were lost to follow-up (Figure 1), in all instances due to inability to contact them after a missed scheduled appointment. At five years, the primary endpoint of MACE had occurred in 12.5% and 17.9% of the SES and BES groups, respectively (p=0.4; Figure 2 and Table 2). Both stents presented low rates of repeat revascularization during the follow-up period, with only around 5% of patients needing to be retreated after five years. Though not statistically significant, the group treated with SES tended to have a numerically lower rate of MI. Of note, there was no definite or probable stent thrombosis among patients treated with the novel SES stent during the five years of follow-up, and no stent thrombosis after the first year in the BES group.
Clinical outcomes at five years of follow-up.
| SES (n=114 patients) | BES (n=56 patients) | p | |
|---|---|---|---|
| 1 year | |||
| Cardiac death | 0.0 | 0.0 | - |
| MI | 4.4 | 7.4 | 0.5 |
| Target lesion revascularization | 2.7 | 3.7 | 0.7 |
| MACE | 6.3 | 9.4 | 0.5 |
| Definite or probable stent thrombosis | 0.0 | 1.8 | 0.2 |
| 2 years | |||
| Cardiac death | 0.0 | 0.0 | |
| Myocardial infarction | 4.4 | 11.3 | 0.1 |
| Target lesion revascularization | 2.7 | 5.6 | 0.4 |
| MACE | 6.3 | 13.3 | 0.2 |
| Definite or probable stent thrombosis | 0.0 | 1.8 | 0.2 |
| 3 years | |||
| Cardiac death | 2.8 | 1.9 | 0.7 |
| Myocardial infarction | 5.4 | 14.5 | 0.1 |
| Target lesion revascularization | 4.6 | 5.4 | 0.8 |
| MACE | 10.0 | 16.3 | 0.2 |
| Definite or probable stent thrombosis | 0.0 | 1.8 | 0.2 |
| 4 years | |||
| Cardiac death | 3.8 | 1.9 | 0.5 |
| Myocardial infarction | 6.3 | 14.5 | 0.1 |
| Target lesion revascularization | 4.6 | 5.4 | 0.8 |
| MACE | 12.0 | 16.3 | 0.4 |
| Definite or probable stent thrombosis | 0.0 | 1.8 | 0.2 |
| 5 years | |||
| Cardiac death | 3.8 | 1.9 | 0.5 |
| Myocardial infarction | 6.5 | 15.4 | 0.1 |
| Target lesion revascularization | 4.6 | 5.6 | 0.8 |
| MACE | 12.5 | 17.9 | 0.4 |
| Definite or probable stent thrombosis | 0 | 1.9 | 0.14 |
Numbers are cumulative hazard ratios.
BES: biolimus-eluting stent; MACE: major adverse cardiac events (cardiac death, myocardial infarction, or target lesion revascularization); MI: myocardial infarction; SES: sirolimus-eluting stent.
The main finding of the present study was that patients treated with the Inspiron™ sirolimus-eluting stent had good outcomes at five years, comparable to the control Biomatrix™ Flex biolimus-eluting stent. Importantly, no cases of definite or probable stent thrombosis were associated with either stent after the first year of follow-up.
The low rates of adverse events observed in the DESTINY trial are noteworthy, in the SES arm as well as in the control BES arm. This low frequency of complications may be related to the relatively low-risk profile of the included population. To compare, in the LEADERS trial of all-comers treated with a BES similar to the control stent used in DESTINY, the five-year rate of all-cause death, any MI, or all-cause revascularization was 35.1%.3 Nevertheless, the favorable results found in our study are in line with several other trials of DES with biodegradable polymer coatings, in which the five-year rates of combined events ranged between 10.0% and 13.4%.10–13
Both study stents are abluminally coated with biodegradable polymers that, theoretically, undergo full degradation by 6-9 months, after which the remaining implant should resemble a bare-metal stent.7 Previous data from the DESTINY trial had shown that both study stents are associated with excellent inhibition of neointimal growth and clinical performance in the first months after implantation.6,7 The present study addresses the central issue of the safety and efficacy profiles of the stents beyond the first year, after degradation of the polymer coating.
The low rates of repeat revascularization seen in the present report, consistently in both arms across the five-year follow-up period, strongly suggests that the two study stents are associated with long-term efficacy long after implantation. Equally important, both SES and BES were associated with zero stent thrombosis after the first year, highlighting the superior safety behavior of both stents up to five years.
LimitationsWe should emphasize that the present study has limitations. The trial was initially designed to assess non-inferiority between the two stents regarding angiographic findings. Although we herein report a predefined subsidiary analysis, the present results should be analyzed with caution. Most of the limitations are related to the small patient sample, which can intrinsically bias the analysis of infrequent events, such as very late stent thrombosis. Nevertheless, the zero very late thrombosis rate is reassuring and strongly indicates a good safety profile. The relatively low complexity of clinical and angiographic features of our cohort may have influenced the favorable results found in the present work, which cannot be directly extrapolated to other subsets. Nevertheless, the novel Inspiron™ stent, as well as the comparator Biomatrix™ stent, have both been tested in scenarios of higher complexity, in which they also showed good clinical performance.3,14
ConclusionThe Inspiron™ stent, an ultrathin-strut, low-dose, sirolimus-eluting device with abluminal-only biodegradable polymer coating, had similar good clinical performance in long-term follow-up when compared head-to-head with the control latest-generation Biomatrix™ Flex biolimus-eluting stent.
FundingThe study was sponsored by Scitech Medical (Aparecida de Goiania, Brazil). The company contributed to study design but had no access to the raw data and no role in conducting the trial (data collection and monitoring) as well as no role in data analysis, interpretation, or writing of the manuscript.
Conflicts of interestDr. Prado Jr reports consulting fee from Scitech; Dr. Chamie has received consulting fees from St. Jude Medical; the other authors have no conflicts to declare.