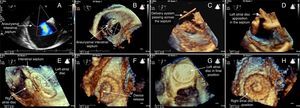
We report the case of a 70-year-old woman treated by percutaneous closure of ostium secundum atrial septal defect (ASD), associated with aneurysm of the atrial septum.
The procedure was performed under general anesthesia, using fluoroscopic and two- (2D) and three-dimensional (3D) transesophageal echocardiography (TEE) imaging. There was a large ASD, with an aneurysmal component on the posterior border. It is frequently difficult to measure the size of an ASD by 2D echocardiography in such patients. When the septal aneurysm, with poor support, is excluded from the measurement, only the thicker septum is considered, oversizing the ASD and device, with increased risk of erosion. On the other hand, inclusion of the aneurysmal wall as part of the supporting border will reduce the measured diameter, with increased risk of embolization. We used 3D TEE to precisely determine the defect size at two perpendicular diameters (13 mm×19 mm) and the length of the aneurysmal wall with potential support for a device (Figure 1A and B). We implanted an 18 mm CeraFlex™ ASD Occluder (Lifetech Scientific, Shenzhen, China), using a standard technique. The system was positioned across the septum (Figure 1C). After delivery, 3D TEE confirmed the apposition of the two discs of the device on the AS wall and a suitable final position (Figure 1D and E), particularly for the inferior and posterior border, frequently difficult with an aneurysmal septum. Finally, the device was released (Figure 1F).
This case highlights the use of an advanced echocardiographic technique in percutaneous ASD closure with an aneurysm of the atrial septum, with an excellent result (Figure 1G and H).
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.