Percutaneous coronary intervention (PCI) has been increasingly performed in patients with severely depressed left ventricular function and complex coronary lesions, including multivessel disease.
Mechanical ventricular assist devices play an increasingly important role in high-risk PCI. Impella CP® (Abiomed, Inc.) is a new percutaneous left ventricular assist device, designed for short-term circulatory support. It is a promising option for hemodynamic support in high-risk procedures and can potentially reduce PCI-related complications.
The authors present two case reports of high-risk PCI using the Impella CP® device.
In the setting of low coronary flow reserve, severely depressed left ventricular function and potential hemodynamic instability, the Impella CP® device has made it possible to maintain hemodynamic stability during procedures, without being associated with vascular complications.
As intervenções coronárias percutâneas (ICP) têm sido feitas com maior frequência em doentes com depressão severa da função ventricular esquerda e com lesões coronárias complexas, inclusive doença coronária multivaso.
Os dispositivos de assistência ventricular mecânica desempenham um papel cada vez mais importante nas ICP de elevado risco. O Impella CP® (Abiomed, Inc.) é um novo dispositivo percutâneo de assistência ventricular mecânica esquerda, desenvolvido para apoio circulatório de curta duração. Constitui uma opção prometedora de apoio hemodinâmico em procedimentos de elevado risco, com o potencial de reduzir as complicações relacionadas com as ICP.
Os autores apresentam dois casos clínicos de ICP de alto risco que usam o dispositivo Impella CP®.
No contexto de baixa reserva coronária, depressão severa da função do ventrículo esquerdo e de potencial instabilidade hemodinâmica, o Impella CP® permitiu manter a estabilidade hemodinâmica dos doentes durante os procedimentos, sem se associar a complicações vasculares.
coronary artery bypass grafting
cardiac magnetic resonance imaging
left anterior descending
left circumflex
left main coronary artery
left ventricle
left ventricular ejection fraction
mechanical circulatory support
first obtuse marginal
percutaneous coronary intervention
right coronary artery
saphenous vein graft
transthoracic echocardiogram
unfractionated heparin
Percutaneous coronary interventions (PCIs) have been increasingly performed for the treatment of stenotic coronary artery disease. In Portugal alone, the number of PCIs performed increased by 65% in the decade from 2004 to 2013.1,2 Procedures have become more complex and challenging for the operator, especially with high-risk patients deemed ineligible for surgical revascularization.3 There is no universally accepted definition for so-called “high-risk PCI”, but the term covers the performance of a coronary intervention in high-risk coronary anatomy or in high-risk patients. These patients often have low physiological reserve that may not withstand the deleterious effects of transient ischemia or hemodynamic instability, nor the systemic impact of ischemia-reperfusion injury or no-reflow phenomena.
In this setting, the use of mechanical circulatory support (MCS) devices may help to avoid clinical deterioration by (1) increasing coronary and peripheral circulation and (2) unloading the myocardium, thereby reducing its work and secondary ischemia.4 The Impella® device (Abiomed, Inc.) is an MCS system that consists of a pump placed percutaneously across the aortic valve under fluoroscopic guidance. It is connected to a console that enables external monitoring, purging and control of the overall system. In short, Impella® aspirates blood from the left ventricle (LV) into the ascending aorta, thereby unloading the LV, increasing aortic and intracoronary pressure and reducing end-diastolic wall stress. The end result is a favorable effect on the supply–demand equilibrium of the myocardium at risk.5 The PROTECT II6 trial is the largest randomized trial comparing Impella® (using Impella 2.5®) with an intraortic balloon counterpulsation to support nonemergent high-risk PCI. The trial found no significant difference in major adverse events at discharge or 30 days, but the per-protocol analysis revealed a strong trend toward decreased adverse events with Impella® at 90 days. Therefore, the use of Impella® during high-risk PCI may hold promise over the long term. Notably, in the Balloon Pump-Assisted Coronary Intervention Study, an intraortic balloon counterpulsation pump failed to reduce major adverse cardiac and cardiovascular events in nonemergent high-risk PCI procedures compared to the conventional strategy.7
Impella CP® (Abiomed, Inc.) is the new generation of the percutaneous, catheter-based device that can provide a flow of up to 3.5 l/min. Here, we present two cases of high-risk PCI performed in our center with LV assistance provided by an Impella CP®.
Case 1A 70-year-old diabetic male with a history of chronic kidney disease (creatinine clearance 43 ml/min) was admitted to our cardiac intensive care unit with non-ST-elevation myocardial infarction, Killip class III. Thirty years previously, he had undergone 3-vessel coronary artery bypass grafting (CABG) with saphenous vein grafts (SVGs) and had subsequently undergone PCI of the SVGs to the first obtuse marginal (OM1) in 2002 and 2009, with implantation of a 4.5×13-mm Bx Velocity™ stent (Johnson & Johnson) and a 4.0×2.8-mm Xience stent (Abbott Vascular), respectively.
On admission, a transthoracic echocardiogram (TTE) showed severely depressed left ventricular ejection fraction (LVEF) (estimated 29%); akinesis of the apex, inferior wall, middle and distal segments of the anterior wall; and hypokinesis of the other walls. There was no evidence of intracavitary thrombi. Invasive coronary angiography revealed severe native coronary artery disease consisting of an occluded left anterior descending (LAD) artery at the origin and occluded left circumflex (LCX) and right coronary artery (RCA) in their middle segments. The SVG to LAD was occluded, the SVG to the OM1 had 90% stenosis proximally (pre-stent) and the SVG to the right posterior descending artery had 70% stenosis distally. The patient underwent cardiac magnetic resonance imaging (CMRI), which showed extensive myocardial scarring involving the apex, the inferior wall and the middle and distal segments of the anterior wall. Finally, an adenosine stress perfusion CMRI revealed ischemia of the basal segment of the inferolateral wall. Based on this evidence, and in an attempt to improve the patient's status, it was decided to perform a PCI of the SVG to the OM1. A 4-l/min Impella CP® assist device was selected for support due to the low physiologic reserve of the patient and the relevance of the aforementioned graft in the perfusion of the remaining viable myocardium.
Following local anesthesia, a 6-Fr sheath was inserted into the right femoral artery. After performing a contrast study of the aorta and both iliac arteries, the patient was considered a candidate for using the assist device. A 14-Fr sheath was then placed in the left femoral artery. After administration of 5000 units of unfractionated heparin (UFH), an angiographic pigtail diagnostic catheter (Cardinal Health) was used to deliver a specific 0.14-inch guidewire to the LV. The diagnostic catheter was then removed, and the Impella CP® pump was advanced over the wire across the aortic valve under angiographic guidance (Figure 1A). The pump was started, with a maximum of 3.5 l per minute of circulatory support necessary to maintain the patient hemodynamically stable during the procedure.
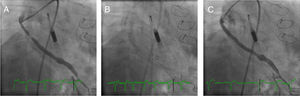
Case 1. (A) The right anterior oblique caudal view reveals the saphenous vein graft (SVG) to the first obtuse marginal before intervention and the Impella CP® device. (B) Distal embolic protection device, Emboshield NAV (Abbott Vascular), deployed in the distal portion of the SVG. (C) The right anterior oblique caudal view shows the final angiographic result.
The SVG to the OM1 was intubated with a 6-Fr Amplatz Left 1 catheter (Cardinal Health). A distal embolic protection device, the 6-Fr Emboshield NAV (Abbott Vascular), was gently passed into the SVG across the target lesion and was deployed at the distal portion of the graft (Figure 1B). After pre-dilation of the target lesion with a 3.5×20-mm TREK balloon (Abbott Vascular), a 4.0×28-mm Xience stent was successfully deployed in the SVG. Next, the stent was post-dilated with a 4.5×15-mm Quantum™ Maverick™ balloon (Boston Scientific) at 18 atm. The Emboshield NAV basket was then carefully withdrawn. Aspiration was performed to clear the guide catheter of any debris and thrombi and ensure that none of the contents of the basket remained in the guiding catheter (Figure 1C).
The intervention was uneventful and the patient remained hemodynamically stable throughout the procedure. The Impella CP® assist device was withdrawn at the end of the procedure and the left femoral artery access site was transcutaneously closed with two ProGlide (Abbott Vascular) devices, a suture-mediated closure system with threads made of non-resorbable 3-0 polypropylene. Vascular closure of the right femoral artery was achieved with Angio-Seal™ (St. Jude Medical), a device that employs a collagen plug that not only physically closes the arteriotomy site but also induces platelet activation and aggregation when deployed.
The patient was safely discharged from hospital two days later. After eight months, he reported class II angina according to Canadian Cardiovascular Society grading system and mild episodes of dyspnea on moderate effort.
Case 2An 80-year-old male with type 2 diabetes, hypertension, dyslipidemia and a permanent pacemaker device implanted one year before due to complete heart block was transferred to our hospital with an acute myocardial infarction, Killip class III.
An electrocardiogram obtained immediately upon admission showed stable right ventricular pacing rhythm. TTE showed a severely depressed LVEF (34%) with global hypokinesis of the left ventricle. There was no evidence of intracavitary thrombi. Coronary angiogram revealed 70% stenosis of the distal left main coronary artery (LMCA) (Medina Classification 1,0,1) and chronic total occlusions of the proximal LCX and middle RCA, both partially perfused by collateral vessels (Figure 2A). The patient was rejected for CABG because of high operative risk and poor distal grafting targets. After a Heart Team discussion, it was decided to proceed with a PCI of the distal LMCA to the LAD with the support of a 4-l/min Impella CP®.
The procedure started with the insertion of a 6-Fr sheath into the right radial artery. A contrast study of the aorta and both iliac arteries showed that the patient was a candidate for using the ventricular assist device. A 14-Fr sheath was then inserted into the right femoral artery and, after administration of 5000 units of UFH, the 4-l/min Impella CP® assist device was placed into the left ventricle, as previously described. The device was started, with a maximum of 3.0 l/min of circulatory support necessary to maintain the patient hemodynamically stable during the procedure.
The ostium of the LMCA was catheterized using a 6-Fr Extra Back-Up 3.5 guiding catheter (Cardinal Health) via right radial artery. A Whisper (Abbott Vascular) guidewire was advanced into the LAD and multiple pre-dilation inflations were performed using a 2.5×8-mm Quantum™ Maverick™ balloon and a 3×8-mm TREK balloon. Then a 3.5×18-mm Onyx™ (Medtronic) stent was successfully deployed in the distal LMCA towards the proximal LAD. The stent was subsequently post-dilated with a 4.0×15-mm Quantum™ Maverick™ balloon at 20 atm, with a good final angiographic result (Figure 2B). The patient remained hemodynamically stable throughout. The Impella CP® assist device was withdrawn and the sheath was removed at the end of the procedure. The right femoral artery access site was closed using a ProGlide device.
The patient experienced a favorable clinical course and was discharged from hospital four days after the procedure. Five months later, he was free of chest pain and reported class II dyspnea according to the New York Heart Association Functional Classification.
ConclusionIn this manuscript, we presented two cases of high-risk PCI using the Impella CP® device. In the setting of low coronary reserve, severely depressed LV function and potential hemodynamic instability, this device enabled hemodynamic stability to be maintained during the procedures without increasing vascular complications.
Author contributionsSara Moura-Ferreira: Drafted and designed the article; acquired, analyzed and interpreted data; and approved the submitted and final versions.
Ricardo Ladeiras-Lopes: Drafted and designed the article; acquired, analyzed and interpreted data; and approved the submitted and final versions.
Domingas MBala: Performed a detailed analysis and interpretation of data; provided a critical review of the article; and approved the submitted and final versions.
Alberto Rodrigues: Performed a detailed analysis and interpretation of data; provided a critical review of the article; and approved the submitted and final versions.
Pedro Braga: Performed a detailed analysis and interpretation of data; provided a critical review of the article; and approved the submitted and final versions.
Vasco Gama: Contributed to data collection and interpretation; provided a critical review of the article; and approved the submitted and final versions.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors gratefully acknowledge Gustavo Pires-Morais, Lino Santos and Bruno Melica for providing additional insightful critical reviews of this paper.





 SVG) to the first obtuse marginal before intervention and the Impella CP® device. (B) Distal embolic protection device, Emboshield NAV (Abbott Vascular), deployed in the distal portion of the
SVG) to the first obtuse marginal before intervention and the Impella CP® device. (B) Distal embolic protection device, Emboshield NAV (Abbott Vascular), deployed in the distal portion of the 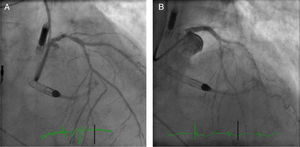 LMCA) stenosis and the Impella CP® device. (B) The right anterior oblique cranial projection after distal-LMCA/proximal-left anterior descending percutaneous coronary intervention.'/>
LMCA) stenosis and the Impella CP® device. (B) The right anterior oblique cranial projection after distal-LMCA/proximal-left anterior descending percutaneous coronary intervention.'/>

