Hypoplastic left heart syndrome (HLHS) is a major cause of cardiac death during the first week of life. The hybrid approach is a reliable, reproducible treatment option for patients with HLHS. Herein we report our results using this approach, focusing on its efficacy, safety and late outcome.
MethodsWe reviewed prospectively collected data on patients treated for HLHS using a hybrid approach between July 2007 and September 2014.
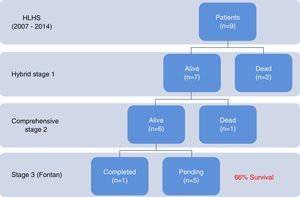
ResultsNine patients had a stage 1 hybrid procedure, with seven undergoing a comprehensive stage 2 procedure. One patient completed the Fontan procedure. Five patients underwent balloon atrial septostomy after the hybrid procedure; in three patients, a stent was placed across the atrial septum. There were three deaths: two early after the hybrid procedure and one early after stage two palliation. Overall survival was 66%.
ConclusionsIn our single-center series, the hybrid approach for HLHS yields intermediate results comparable to those of the Norwood strategy. The existence of dedicated teams for the diagnosis and management of these patients, preferably in high-volume centers, is of major importance in this condition.
A síndrome do coração esquerdo hipoplásico (SCEH) é uma das principais causas de morte durante a primeira semana de vida. A abordagem híbrida é uma opção de paliação para doentes com SCEH. Reportamos os nossos resultados com esta abordagem, com foco particular na sua eficácia, segurança e resultado final.
MétodosTrabalho prospetivo. Revisão dos dados clínicos de doentes com SCEH, submetidos a abordagem híbrida de paliação entre julho de 2007 e setembro de 2014.
ResultadosNove doentes foram submetidos a primeiro estadio híbrido de paliação. Destes, sete completaram segundo estadio e um doente foi submetido a cirurgia de Fontan. Cinco doentes foram submetidos a atriosseptostomia com balão. Em três procedeu-se a implantação de stent no septo interauricular. Verificaram-se três óbitos: dois logo após o primeiro estadio híbrido e um após o segundo estadio. A sobrevida global foi de 66%.
ConclusõesNa nossa experiência, a abordagem híbrida para SCEH produz resultados comparáveis aos da estratégia de Norwood. A necessidade de uma equipa dedicada para o diagnóstico e manejo destes doentes, de preferência em centros de alto volume, é de grande importância nesta condição particular.
Hypoplastic left heart syndrome (HLHS) represents 1.4–3.8% of congenital heart diseases but is responsible for 23% of cardiac deaths during the first week of life.1–3 HLHS is characterized by variable degrees of underdevelopment of left heart structures.4–6 Traditional management of HLHS, either with staged surgical palliation (Norwood, Glenn, Fontan)7,8 or cardiac transplantation,9 remains a challenge in most centers, particularly in high-risk patients such as those with low birth weight (<3 kg) and diminutive aortas (<2 mm).10–12 For this subgroup of patients, a hybrid approach – combining transcatheter and surgical techniques – was devised in the last decade, aiming to combine the best characteristics of surgical and interventional cardiology techniques.13–15
Originally reported in 1993,16 hybrid stage 1 palliation consists of bilateral branch pulmonary artery banding and stenting of the ductus arteriosus, without cardiopulmonary bypass, during the neonatal period. Assurance of a nonrestrictive atrial septal defect is the next step, through either balloon atrial septostomy or stenting. Later in infancy, a more complex comprehensive stage 2 is performed at approximately six months of age, in the form of a Norwood-type reconstruction combined with a bidirectional cavopulmonary (Glenn) anastomosis. Although initially suggested for high-risk candidates for the classic Norwood stage 1 and 2 palliation,14,17 it has since been adopted as the preferred first option by several centers.14,18,19
In 2007, our center started a hybrid program for HLHS palliation in high-risk cases. Our aim is to report our initial results with this technique, focusing on its efficacy, safety and late outcome, with particular emphasis on morbidity and mortality, need for unplanned reintervention, and the current status of the patients.
MethodsPatient populationOur cohort comprised patients who underwent a hybrid stage 1 procedure between July 2007 and September 2014. All had typical HLHS (aortic atresia or critical stenosis with mitral atresia or stenosis). Initially, our center chose only high-risk patients for the hybrid palliation, defined by the presence of low birth weight (<2.5 kg), prematurity, severe aortic arch hypoplasia, poor right ventricular function, more than mild tricuspid regurgitation, highly restrictive atrial septal defect, and the presence of non-cardiac malformations. Later on, we widened our criteria and opted for a hybrid approach for all HLHS patients. A review of prospectively collected data including information from all planned staged procedures, any unplanned reinterventions, and interstage morbidity and outcomes are discussed herein. Follow-up was complete in all patients (Figure 1).
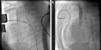
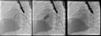
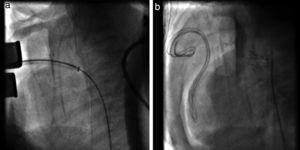
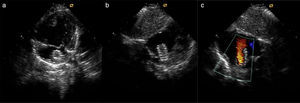
TechniqueHybrid stage 1The procedure was performed in the catheterization laboratory, adapted as a hybrid suite, under general anesthesia. The technique was based on reports by Galantowicz et al.18 Briefly, through a median sternotomy, and without cardiopulmonary bypass, bilateral branch pulmonary artery (PA) bands were placed using a 3.5-mm Gore-Tex tube graft (WL Gore & Associates, Flagstaff, AZ, USA); the band was first placed on the left PA. The circumference of the band was calculated according to the caliber of the branch PA and the degree of band tightening was adjusted according to the response of systemic blood pressure and oxygen saturation (O2 Sat), aiming for O2 Sat percentages in the high 70s to low 80s. After bilateral pulmonary banding a sheath was inserted through a controlled arteriotomy directly in the main pulmonary artery and a balloon-expandable stent (Genesis, Cordis, Johnson & Johnson, Miami, FL, USA) was deployed to completely cover the ductus arteriosus (Figures 2 and 3). Whenever there was evidence of a restrictive interatrial septal defect (ASD) (mean Doppler gradient by echocardiography ≥8 mmHg), it was relieved in a separate procedure,20 either interventional (Figures 4 and 5) or surgical.
After discharge, patients were closely monitored every one to two weeks with serial O2 Sat and weight measurements and with complete cardiology assessments: echocardiograms were performed to monitor for obstruction of pulmonary venous return at the atrial septum or obstruction to aortic arch blood flow (antegrade, retrograde or through the patent ductus arteriosus stent). Decreased right ventricular function or increased atrioventricular valve regurgitation were considered early markers of increased afterload. Patients were maintained on chronic afterload reduction with an angiotensin-converting inhibitor plus diuretics and digoxin. Prophylactic antiplatelet therapy with aspirin 3–5 mg/kg/day was uniformly administered.
Comprehensive stage 2Patients underwent comprehensive stage 2 surgery at six to nine months of age, depending on clinical assessment. Cardiac catheterization was electively performed in all cases prior to the comprehensive stage 2 procedure to obtain diagnostic data (such as assessment of physiology or associated anomalies) and/or to relieve any obstruction (systemic or at the atrial septum).
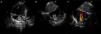
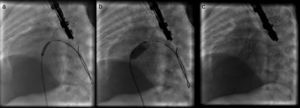
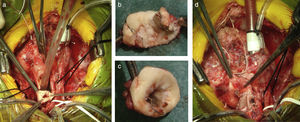
The comprehensive stage 2 surgery consisted of debanding of the branch pulmonary arteries, resection of the stented ductus arteriosus, reconstruction of the aortic arch and pulmonary arteries (if needed), division of the diminutive ascending aorta with reimplantation into the main PA to reconstruct systemic outflow (a Norwood-like procedure – Figure 6), atrial septectomy, and construction of a bidirectional cavopulmonary anastomosis (Glenn procedure). The right pulmonary band site was incorporated in the Glenn shunt and enlarged if needed; a left pulmonary patch enlargement was performed when judged necessary.
Stage 3The total cavopulmonary circulation (stage 3 surgery) was completed with an extracardiac fenestrated conduit connecting the inferior vena cava to the pulmonary arteries via a Gore-Tex conduit (WL Gore & Associates, Elkton, Maryland).
ResultsHybrid stage 1The hybrid stage 1 was performed on nine patients with HLHS at a median age of 9.5 days and weight 3 kg. Patient characteristics are listed in Table 1. Only two patients received blood products. Most (84%) were weaned from ventilation on the second postoperative day (median two days; range 2–15 days). Median length of stay in the intensive care unit was 9.5 days (range 3–42 days). The median time to first enteral feed was postoperative day 2 (range 2–20 days). No patient experienced infectious complications, necrotizing enterocolitis or acute renal failure. There was 77% hospital survival (7 out of 9). During this period two patients died after stage 1 palliation with low cardiac output syndrome: one due to acute ductus arteriosus occlusion, because of incomplete ductal stenting, unresponsive to prostaglandin infusion and unable to be taken back to the catheterization laboratory for placement of an additional coaxial ductal stent; and the other due to stent-related retrograde aortic arch obstruction. Postoperative outcomes are shown in Table 2.
Diagnosis and clinical profile.
| Diagnosis | Age 1 (days) | Weight 1 (kg) | Ascending aorta (mm) | Stage 2 | Age 2 (months) | Weight 2 (kg) | Stage 3 | Current status | Stage of death | Cause of death | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | AA/MA | 9 | 2.4 | 2.3 | Yes | 8 | 6.3 | Yes | Alive | ||
| 2 | AA/MA | 18 | 3.3 | 2.7 | Yes | 9 | 6.6 | Pending | Alive | ||
| 3 | AA/MS | 14 | 3 | 3 | Yes | 9 | 6.3 | No | Dead | 2 | LCOS |
| 4 | AA/MA | 10 | 3.23 | 2 | No | No | Dead | 1 | Retrograde aortic arch obstruction | ||
| 5 | AA/MA | 90 | 3.43 | 2 | Yes | 9 | 6 | Pending | Alive | ||
| 6 | AA/MA | 5 | 3 | 2 | Yes | 9 | 6.9 | Pending | Alive | ||
| 7 | AA/MS | 7 | 2.6 | 2.3 | No | No | Dead | 2 | Incomplete ductal stenting | ||
| 8 | AA/MA | 9 | 3.1 | 2.6 | Yes | 8 | 6.1 | Pending | Alive | ||
| 9 | AS/MS | 12 | 2.4 | 3 | Yes | 7 | 6 | Pending | Alive | ||
| Mean | 20.25 | 3.01 | 2.36 | 8.4 | 6.37 | ||||||
| Median | 9.5 | 3.05 | 2.3 | 9 | 6.3 | ||||||
AA: aortic atresia; AS: aortic stenosis; LCOS: low cardiac output syndrome; MA: mitral atresia; MS: mitral stenosis.
Hybrid stage 1 results.
| Intervention | Time to extubation (days) | Time to enteral feeding (days) | ICU stay (days) | LOS (days) | |
|---|---|---|---|---|---|
| 1 | PAB + PDA stent | 2 | 2 | 6 | 22 |
| 2 | PAB + PDA stent | 2 | 13 | 13 | 150 |
| 3 | PAB + PDA stent | 2 | 2 | 14 | 60 |
| 4 | PAB + PDA stent | 0 | 0 | 2 | 2 |
| 5 | PAB | 2 | 2 | 3 | 19 |
| 6 | PAB + PDA stent | 2 | 2 | 13 | 26 |
| 7 | PAB + PDA plasty | 0 | 0 | 3 | 3 |
| 8 | PAB + PDA plasty | 15 | 20 | 42 | 85 |
| 9 | PAB + PDA stent | 2 | 2 | 4 | 20 |
| Mean | 3.125 | 5.125 | 12 | 45.875 | |
| Median | 2 | 2 | 9.5 | 24 | |
ICU: intensive care unit; LOS: length of stay; PAB: pulmonary artery banding; PDA: patent ductus arteriosus.
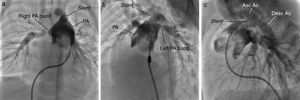
Balloon atrial septostomy or atrial septectomy was performed as a separate procedure after the hybrid stage 1. Five patients underwent balloon atrial septostomy at a median of 10 days after the hybrid procedure; in two of these, due to increasingly restrictive atrial septal flow, a surgical atrial septectomy was later performed at 26–47 days after septostomy. In three patients, due to an unusually thick atrial septum with a superiorly positioned atrial septal defect, making balloon septostomy difficult, an 8 mm×15 mm stent (Palmaz Genesis, Cordis, Johnson & Johnson, Miami, FL, USA), was placed across the atrial septum (Figures 4 and 5). One of these patients underwent emergent atrial septectomy and stent removal due to stent migration to the right atrium.
Interstage 1–2There were no interstage deaths. Prior to the comprehensive stage 2, the echocardiographic assessment of right ventricular function was graded as normal in all but two patients, in whom it was graded as mildly depressed. Tricuspid regurgitation was trivial in all but two patients.
There were reinterventions in the catheterization laboratory (balloon angioplasty) in two patients due to recoarctation, both successful. All patients proceeded to a comprehensive stage 2.
Comprehensive stage 2A comprehensive stage 2 procedure was performed in seven patients at a median age of nine months and 6.3 kg weight. Median bypass and cross-clamp times were 284 and 90 min, respectively. Low dose inotropic support (dopamine or milrinone) was used in the early post-operative period. No patient required a delayed sternal closure. The postoperative echocardiographic assessment of right ventricular function was graded as normal in 80%, with only one patient having greater than mild dysfunction or greater than mild tricuspid regurgitation. Four of six patients were extubated within 24 hours (median 25 hours; maximum 32 hours). Median lactate level was 20 mg/dl (range 10–35 mg/dl) on arrival at the ICU, and 10 mg/dl (range 7–22 mg/dl) on postoperative day 1. The median length of stay in the ICU was seven days (range 4–16 days). No patient had renal failure. All patients were in normal sinus rhythm and there were no arrhythmias.
One patient out of seven died after comprehensive stage 2 palliation (14% mortality). The patient died on postoperative day 1 from low cardiac output syndrome and Glenn obstruction.
Fontan completionOne patient underwent a successful Fontan completion with no mortality, while five others are awaiting Fontan completion; survival to completion of stage 2 palliation is 66% of the patients (six out of nine).
DiscussionWe report our initial results with the hybrid approach for palliation of patients with HLHS. These results reflect our technical challenges and learning curve.
In recent years, several strategies have had a noticeable impact on the disease burden of HLHS, most notably the right ventricle to pulmonary artery Sano modification21 and the development of dedicated teams for close monitoring of HLHS patients between stages 1 and 2 of palliation.22 It has been shown, however, that there are subgroups of patients within the spectrum of HLHS which have poorer outcomes when treated by traditional surgical palliation.10,23 Also, the classical staged palliation approach appears to have reached a stagnation point,24 no further improvements being possible with the currently available technology, with recent cumulative early and interstage mortality of 5%–30% for standard-risk patients25–27 but as high as 30%–50% in high-risk patients.11,12 A recent Pediatric Heart Network-sponsored multicenter randomized trial compared the Norwood procedure with a modified BT shunt versus the Sano modification,26 with the primary endpoint of death or transplantation at one year and the secondary endpoints of hospital course, RV function by echo, pulmonary artery size by angiography, unintended cardiovascular interventions, and serious adverse events and complications. This study failed to show clear superiority between any of the approaches in the long term. Although the Sano modification group was found to have a statistically significant lower risk of mortality at the 12-month endpoint, this was no longer significant with a longer follow-up. The complication rate was also higher in the Sano group, although the percentage of infants with at least one complication was the same in both groups.26
Hybrid palliation has evolved as an alternative to Norwood palliation based on the assumption that a less invasive stage 1 palliation in the hybrid strategy would improve overall survival and neurological outcomes.28 The goals of hybrid stage 1 palliation include the following29: (1) to ensure unimpeded systemic cardiac output through a patent ductus arteriosus; (2) to obviate the need for and complications associated with long-term prostaglandin E1 (PGE1) infusion; (3) to improve balance of the pulmonary and systemic circulations by restricting pulmonary blood flow; and (4) to relieve any obstruction to pulmonary venous return at the atrial septum level. One of the most important advantages of hybrid palliation is to defer the most complex surgical intervention to later in infancy, reducing the risk of neurological complications. In our series the majority of patients were extubated in the first 48 hours and were discharged home after a median hospital stay of 24 days, significantly shorter than for the traditional Norwood procedure, and have a normal neurological assessment to date.
The hybrid approach is also more flexible than the conventional staged approach.30 Some authors have even modified the hybrid approach and propose a partial hybrid stage I palliation in the form of bilateral PA banding with continuous PGE1 administration.31 It may be used as a bailout or salvage approach,32 providing excellent palliation for patients for whom transplantation is considered the most appropriate long-term approach. In such circumstances the time pressure to find a suitable donor heart is relieved and the child is likely to be exposed to fewer blood products, reducing problems with later organ matching. Likewise, some patients with a borderline left ventricle may be palliated using the hybrid approach, allowing further growth or recovery of cardiac function so that a two-ventricle repair may ultimately be attempted.33,34 While it is possible to take down a Norwood stage 1, this is a much larger undertaking than converting a hybrid procedure.
Despite the constant evolution of interventional techniques, the hybrid approach still faces certain limitations. A particularly serious one is the occurrence of retrograde aortic arch obstruction, leading to suboptimal cerebral and coronary artery perfusion.18,15 In our series the two deaths after stage 1 palliation were directly attributable to obstructed systemic cardiac output through the ductus arteriosus. Retrograde aortic arch obstruction can occur in utero, postnatally before surgical treatment begins, or afterward in acute or more insidious fashion after ductus stenting.35 Its incidence ranges from 8% to 30%. Some centers have managed this problem by stenting the stenotic isthmus,36 thus rendering reconstructive arch surgery even more challenging, or by creating a prophylactic reverse Blalock-Taussig shunt,37 while others consider evidence of restricted flow into the retrograde transverse aorta from the ductus arteriosus an exclusion criterion for the hybrid procedure.15 Nevertheless, it is highly desirable to avoid a hybrid procedure in patients with obstruction to retrograde arch blood flow, which leads to poorer results and significantly increased mortality.
Furthermore, several authors argue that the hybrid approach fails to attain some of the essential prerequisites for an optimal Fontan-type circulation, classically described by Choussat and Fontan.38 For these authors specific physiological risk factors for a failing Fontan prevail, related to ventricular performance, atrioventricular and systemic valve function, and pulmonary and systemic circulation. Some of the potential problems reported include: (1) risk of residual aortic arch obstruction, due to either inappropriate stent deployment leaving distal ductal tissue uncovered or to intrastent occlusion15,39; (2) increased single ventricle afterload due to the non-compliant/non-expansible nature of the stent placed in the ductus arteriosus and to excessively tight PA bands that may also (3) fail to provide adequate pulmonary blood flow or to promote adequate PA growth or even40 (4) induce iatrogenic PA stenosis, necessitating PA reconstruction in the comprehensive stage 2 operation41; (5) presence of increasingly restrictive atrial communication, leading to higher pulmonary venous resistance; (6) decreased single-ventricle function (systolic, diastolic or both), due to all the above-mentioned factors and leading to (7) increased atrioventricular valve regurgitation. In our series, prior to the comprehensive stage 2, the echocardiographic assessment of right ventricular function was graded as normal in all but two patients, in whom it was graded as mildly depressed and who had mild to moderate tricuspid regurgitation. These patients had their aortic arch obstruction corrected percutaneously in the interstage cardiac catheterization procedure. In three patients PA reconstruction was deemed necessary. These patients are now waiting for completion of their bicavopulmonary circulation, but our series is still small and lacks a longer follow-up.
In our limited experience, the timing of the atrial interventional procedure is the most difficult decision. In general we tend to perform it around 10 days after the hybrid procedure but, either due to our limited experience or because of morphologically difficult atrial septums, several of our patients needed surgical septectomy.
To obviate these potential limitations, the hybrid technique has evolved during the last decade.15 Several strategies have been proposed, including: (1) deferring the atrial septostomy to a second procedure, immediately before discharge, allowing for a more durable septostomy by using a larger balloon in a larger left atrium; (2) use of cutting and static balloons for the thick atrial septum; (3) use of self-expanding ductal stents; and (4) performing an earlier comprehensive stage 2 procedure to avoid (a) prolonged branch PA banding, promoting enhanced PA growth and obviating the need for branch PA reconstruction; (b) prolonged single-ventricle strain against a non-compliant stent, preserving ventricular function and avoiding atrioventricular valve regurgitation; (c) pulmonary venous return obstruction, so that the patient is in a better condition for the final Fontan operation.
In high-volume centers with modern surgical, anesthetic and intensive care practices, surgical results for average-risk patients born with HLHS to completion of stage 2 palliation are still superior to those offered by the hybrid approach. This is mainly due to mortality during the comprehensive stage 2 surgery, a long and complex operation. For high-risk patients, the results appear to be equivalent, with 50% anticipated survival. Our results are in agreement with reported international series, with survival to completion of stage 2 palliation of 66%. However, there is a learning curve effect that would suggest that results of hybrid palliation will improve with more experience, even for average-risk patients.
In conclusion, there has been considerable progress with the hybrid approach. Though it is relatively early, current international results suggest there may be subgroups of patients that are better palliated with this approach than by surgery. In most institutions surgical results are currently superior for other subgroups of patients. However, as experience in interventional palliation increases, this position will need to be reassessed with multicenter prospective trials comparing both classic and hybrid approaches. Above all, the existence of dedicated teams for the diagnosis and management of these patients, preferably in high-volume referral centers, is of major importance in this condition.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.