A 39-year-old woman underwent uneventful percutaneous occlusion of an ostium secundum atrial septal defect (ASD) with a 22 mm Ultrasept ASD Occluder®. Transesophageal echocardiography (TEE) performed two years after implantation revealed a de novo residual left-to-right shunt through the correctly implanted device. Three-dimensional transesophageal echocardiography (3D TEE) further clarified this finding by showing a perforation of the device membrane coating. The patient underwent transcatheter closure of the residual shunt with a 20 mm Ultrasept PFO® device. The procedure was guided by fluoroscopy and real-time 3D TEE. At the end of the procedure 3D TEE documented correct device deployment with complete defect coverage and absence of residual shunt.
Doente de 39 anos, género feminino, foi submetida com sucesso a encerramento percutâneo de comunicação interauricular do tipo ostium secundum com dispositivo Ultrasept Atrial Septal Defect Occluder® de 22 mm. O ecocardiograma transesofágico, realizado dois anos após implantação, revelou shunt esquerdo-direito residual de novo através do dispositivo corretamente implantado. O ecocardiograma transesofágico tridimensional (ETE3D) objetivou uma perfuração do revestimento do dispositivo. A doente foi submetida a encerramento percutâneo do shunt residual com dispositivo UltraSept PFO Occluder® de 20 mm. O procedimento foi guiado por fluoroscopia e ETE3D. No final do procedimento por ETE3D, foi documentado o correto posicionamento do dispositivo, com completa cobertura da perfuração e ausência de shunt residual.
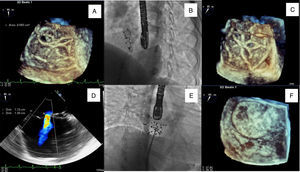
A 39-year-old woman presented for percutaneous closure of an ostium secundum atrial septal defect (ASD) with a balloon-sized diameter of 21 mm, which was performed successfully using a 22 mm Ultrasept ASD Occluder® (Cardia, Eagan, MN, USA) with no complications, under fluoroscopic and transesophageal echocardiography (TEE) guidance. The day after the procedure, transthoracic echocardiography (TTE) showed a correctly placed device with no residual shunt. Two years after the procedure, TTE followed by TEE revealed a residual left-to-right shunt through the correctly implanted device. Three-dimensional (3D) TEE enabled more detailed characterization, showing a perforation of the membrane coating 0.09 cm2 in area on the inferior portion of the device (Figure 1A and D). Although there were no obvious signs of right ventricular overload, the patient complained of fatigue on minimal exertion and occasional chest discomfort.
(A and D) Three- (3D TEE) and two-dimensional transesophageal echocardiography images showing residual shunt through the device (22 mm Ultrasept ASD Occluder®); (B and C) delivery sheath through the Ultrasept device in fluoroscopic view and in 3D TEE; (E and F) closure of residual shunt in the 22 mm Ultrasept device with a 20 mm Ultrasept PFO device, with the final result documented in fluoroscopic view and in 3D TEE.
She accordingly underwent a second cardiac catheterization for hemodynamic measurement and possible transcatheter closure of the residual shunt. The procedure was performed under general anesthesia, guided by fluoroscopy and real-time 3D TEE (Figure 1B, C, E and F). The Qp:Qs obtained by catheterization was 1.7 and therefore we decided to proceed with implantation of a second device. We chose a 20 mm Ultrasept PFO occluder with the expectation that a similar device would conform better to the previously implanted occluder.
The device defect was crossed with a combination of a 6 F right Judkins diagnostic catheter (Boston Scientific, Natick, MA) and a standard 0.35″ ZIPwire™ Hydrophilic Guide Wire (Boston Scientific, Natick, MA) placed in the left superior pulmonary vein. We opted not to perform balloon sizing; otherwise the interventional procedure was performed with the standard ASD closure technique, taking extra care with the 9 F delivery sheath to avoid displacement of the device already in place.
During the procedure, 3D TEE was of crucial importance in guiding the position of guidewires and sheaths, delivery and spatial relations between devices.
The procedure took 60 min with a total radiation time of 14 min and total radiation dose of 865 mGy (8101 cGy.cm2).
At the end of the procedure 3D TEE documented correct device placement with complete defect coverage and absence of residual shunt.
DiscussionThe Ultrasept ASD device is covered by a polyvinyl alcohol (PVA) membrane. PVA is a bioabsorbable elastomeric polymer with good biocompatibility that is commonly used in medical devices due to its low protein adsorption, absence of toxicity and bioadhesive characteristics. Although the use of this material in ASD closure devices is generally successful, a few cases of PVA membrane perforation and recanalization have been described recently.1,2 According to company data only 10 cases have been reported, all of them with ASD occluders and none with PFO closure devices. The mechanism by which the PVA coating degrades is not completely understood, but is likely related to incomplete endothelialization due to delayed or inadequate endothelial response.3 This phenomenon has been reported in a few patients with other ASD devices, such as the Amplatzer™ family, supporting the hypothesis of an absent or inadequate endothelization response in specific patients, of unknown cause.3,4 In our patient, there was no evidence of an early residual shunt, but we cannot speculate on the specific causes that may have given rise to this mid-term residual ASD.
We adopted a percutaneous approach guided by real-time 3D TEE, which offered precise spatial location of the perforation and orientation of sheaths and devices. A 20 mm Ultrasept PFO occluder was chosen because (a) it appeared to be the most easily adjustable for the small but asymmetric hole, (b) no residual holes have been reported in these devices, (c) we thought that it would be better to juxtapose two devices with the same type of frame, and (d) it has a low profile, resulting in a small increase in thickness and no compression of surrounding structures, particularly the aortic wall.
This case highlights the importance of close follow-up in all patients with ASD treated with implanted devices. There should be a low threshold for TEE in the event of any suspicious findings on TTE or changes in the patient's clinical status. Our case demonstrates a new and successful way of correcting ASD device perforations with a percutaneous approach that avoids the need for surgical intervention.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.