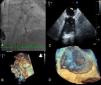
An 81-year-old woman with permanent atrial fibrillation (CHA2DS2-VASc score=5) was referred for left atrial appendage (LAA) closure. She had a history of hypertension, stage IV chronic kidney disease and chronic anemia, secondary to gastrointestinal bleeding (HAS-BLED score=3). Percutaneous LAA closure with a 22 mm first-generation device (Amplatzer™ Cardiac Plug [ACP]) was performed (Figure 1A, Video 1). The procedure was guided by three-dimensional transesophageal echocardiography (TEE) and complete occlusion was confirmed (Figure 1B, Videos 2 and 3). She was treated with aspirin 100 mg plus clopidogrel 75 mg daily for one month, clopidogrel 75 mg daily for six months, and no antithrombotic drug thereafter.
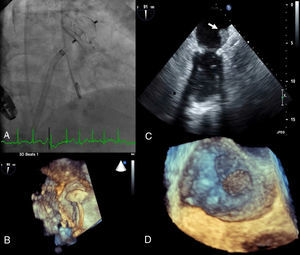
(A) Implantation of a 22 mm Amplatzer™ Cardiac Plug (ACP); (B) three-dimensional transesophageal echocardiography guidance of the procedure; (C) thrombus on atrial surface of the ACP on two-dimensional transesophageal echocardiography (white arrow); (D) three-dimensional transesophageal echocardiography showing thrombus on the device (zoom mode).
By protocol, follow-up TEE was performed at 12 months and revealed a large mobile thrombus attached to the atrial surface of the ACP (Figure 1C and D, Videos 4 and 5).
Warfarin was started and TEE was scheduled for six months later. In the meantime, the patient developed septic shock due to toxic megacolon and died one month after starting warfarin.
Cases of device-related thrombi have been described with the ACP. An early and thorough assessement, either by TEE or angio-computed tomography, should be considered to rule out device thrombosis, especially in first-generation devices, since most thrombi have been documented at 45-day TEE. Newer devices with less thrombotic risk are now available. The optimal duration of antiplatelet therapy and/or a period of oral anticoagulation after device implantation remain to be addressed in future studies.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data is showed in this article.
Right to privacy and informed consentThe authors declare that no patient data is showed in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.