Non-atherosclerotic spontaneous coronary artery dissection (SCAD) is an uncommon but probably underdetected pathological substrate for acute coronary syndrome. Clinical associations have been noted, like female gender and young age, but its pathophysiology is not yet fully understood. In this report we describe the case of a 50-year-old woman, without cardiovascular risk factors presenting with non-ST segment elevation myocardial infarction, in whom SCAD was diagnosed. Treatment was initially conservative but due to aggravation of the dissection she eventually underwent a complex percutaneous coronary intervention, requiring implantation of multiple stents, but with a good clinical outcome. The procedure was guided by optical coherence tomography (OCT). Carefully analyzing the combined pictures of OCT and angiography, the dissection appeared to be filled with a clear fluid, but not contrast.
A disseção espontânea de artéria coronária (DEAC) é uma causa pouco frequente, mas provavelmente sub-diagnosticada, de síndrome coronária aguda. Estão descritas algumas associações clínicas, como o sexo feminino e a idade jovem, mas ainda não está estabelecida a completa fisiopatologia desta entidade. Neste trabalho apresentamos o caso de uma mulher de 50 anos, sem fatores de risco conhecidos, que se apresenta com um enfarte agudo do miocárdio sem supra-desnivelamento de ST, e em quem é diagnosticada uma DEAC. O tratamento foi inicialmente conservador, no entanto, devido a agravamento da disseção, acabou por realizar uma angioplastia complexa, requerendo implantação de vários stents, com bom resultado clínico. O procedimento foi guiado com tomografia de coerência óptica (OCT). Na análise cuidada das imagens de OCT e angiografia, constata-se que a disseção aparenta estar preenchida com um fluido translúcido, mas não contraste.
Spontaneous coronary artery dissection (SCAD) has long been known to the scientific community – at least since the first case description in 1931.1 It is thought to be relatively rare – about 3% of acute coronary syndromes (ACS)2 – but important, since it can have significant consequences such as ischemia, ACS, malignant arrhythmias and sudden cardiac death.3 It may also present with mild and/or atypical symptoms or even be asymptomatic, and can therefore be under-diagnosed. It can also lead to transient wall motion abnormalities and be misdiagnosed as Takotsubo cardiomyopathy.4
The pathophysiology of this entity is not completely understood. Mechanistically, it has been proposed that an intramural hematoma forms due to one of two mechanisms: an intimal tear or intramural hemorrhage from the vasa vasorum.5 There is often an underlying arteriopathy and a precipitating stressful event, like intense exercise. The arteriopathy may be due to atherosclerosis, peripartum state, connective tissue disorders, systemic inflammatory conditions, coronary artery spasm or other conditions.6 It has also been associated with fibromuscular dysplasia in non-coronary arteries.7 Intravascular imaging has a crucial role in the study and management of this condition, especially optical coherence tomography (OCT), due to its very high spatial resolution.8–10
After diagnosis, long-term prognosis is usually benign.11 We report on a clinically challenging case in which a spontaneous dissection was diagnosed.
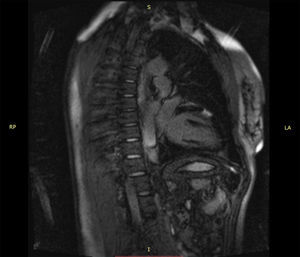
Case reportWe report the case of a 50-year-old woman with no known cardiovascular risk factors and a history of thyroid disease and anemia during adolescence that eventually resolved without a specific diagnosis. She was diagnosed with probable myocardial infarction with normal coronary arteries three years before this event. At that time she had a coronary angiogram, which revealed no angiographically significant coronary artery disease (Video 1). She also underwent a myocardial perfusion scan, which was normal, and cardiac magnetic resonance imaging (MRI), which showed a localized transmural scar in the medial segment of the anterior wall (Online Figure 1). She was discharged on dual antiplatelet therapy for one year, followed by monotherapy with aspirin, as well as a statin, angiotensin-converting enzyme inhibitor and beta-blocker. In May 2015, just starting menopause, and in the context of severe emotional stress, she presented with severe central chest pain radiating to the back and neck, which resolved in 30 min after taking sublingual nitroglycerin. The next day the pain recurred, with greater intensity, sharper (not clearly oppressive) and radiating to both arms, with no relieving factors. She contacted the emergency services and was transported to hospital.
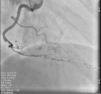
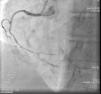
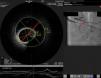
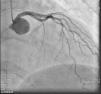
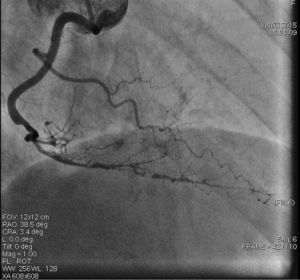
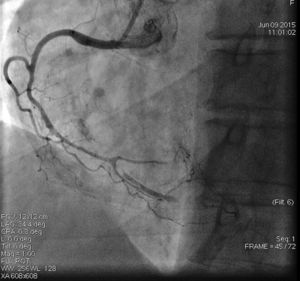
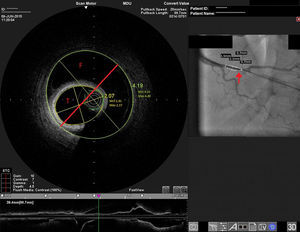
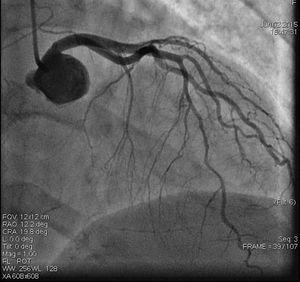
On admission the pain was decreasing; the physical examination was unremarkable, the electrocardiogram showed sinus rhythm, with negative T waves in the inferior leads, and troponin I was positive. The pain eventually disappeared after intravenous nitrates, and she was admitted to the coronary care unit. She underwent coronary angiography (Figure 1, Video 2), which revealed a spontaneous dissection of the posterior descending artery, with TIMI 2 flow, some posterolateral branches visualized from intercoronary collaterals, and severe vasospasm of the proximal right coronary artery (RCA), which reproduced the pain, relieved after intracoronary nitrates. No other unequivocal coronary lesions were noted, although a long dissection of the left anterior descending artery with intact intima cannot be excluded (Online Figure 2). It was decided not to perform percutaneous coronary intervention (PCI), and she was kept in the ward anticoagulated with subcutaneous enoxaparin (1 mg/kg twice daily), with dual antiplatelet therapy and a calcium channel blocker. Screening for autoimmune disease was negative. There were no events during hospitalization and she was scheduled for an angiographic review a week later, which showed progression of the dissection, with a wider false lumen (Figure 2, Video 3). Pain recurred during the procedure and a dissection was noted in a posterolateral branch. It was decided to perform PCI, and two bioabsorbable vascular scaffolds (BVS) were implanted in the PDA, guided by optical coherence tomography (OCT) coregistered with angiography. A third BVS was implanted in the distal RCA due to proximal progression of the dissection. A bare-metal stent was also implanted in the ostial RCA due to a traumatic dissection induced by the guiding catheter. Comparison of the images from angiography and OCT reveals a discrepancy in total (false plus true) diameter in the distal RCA (Figure 3, Video 4). After the procedure the patient was asymptomatic, although with a significant residual dissection in the RCA and posterolateral branch (Video 5). Anticoagulation was suspended and she was discharged five days later, with no further episodes of chest pain. Four months after this episode, she has had no recurrence of pain and is asymptomatic.
Distal right coronary artery (RCA) viewed by optical coherence tomography (OCT). Location of the OCT frame within the RCA is easily identified on the angiogram (arrow). Although the angiogram projection eventually captured the vessel at its smallest diameter (red line), this should be 4.03 mm as measured by OCT. By angiography the largest diameter of the segment is 2.1 mm. This suggests that the false lumen (F) is filled with a clear fluid, but not contrast, since on the angiogram only the true lumen (T) is visible, corresponding to the 2.07 mm mean true luminal diameter measured by OCT. Moreover, on OCT the catheter can be seen at the center of the vessel, but touching the intimal wall, while on the angiogram it appears adjacent to the wall, suggesting that only the true lumen is visible.
This case illustrates several important features of SCAD. Firstly, clinical context: a young woman without cardiovascular risk factors – SCAD may be present in as many as 24% of women aged over 50 with myocardial infarction.12 Secondly, the characteristics of this condition are often elusive, like the often atypical pain (described as “sharp” in this patient). Since she was in the perimenopausal period, hormonal variations may have played a role in this case, possibly similarly to pregnancy-related SCAD.
The first event, three years previously, may also have been a case of dissection, although an intimal flap or intramural hematoma was not visible on careful examination of the images. SCAD may be difficult to diagnose by angiography alone, and can frequently mimic atherosclerosis. A diagnostic algorithm and angiographic classification of SCAD into three types has recently been proposed.13 Intracoronary imaging has a growing role in diagnosing and understanding this condition.
In the recent event, the dissection was evident on angiography, and a conservative approach was adopted, as is common in such cases, with reported good results.14 The patient was on anticoagulants with the purpose of promoting patency of the true lumen, as is the practice in other centers, with good results.15 Despite the patient's clinical stability with absence of pain, in the second procedure an aggravation of the dissection was noted, with progression in length and width of the false lumen. There are no specific recommendations on antithrombotic treatment in these cases and there is some controversy in balancing the risks of true lumen patency and false lumen progression.16 In this case anticoagulation may have had a deleterious effect on intimal stabilization.
Although asymptomatic, the noticeable progression of the dissection prompted treatment with stent implantation. There is little evidence on the use of BVS for the treatment of SCAD, but bearing in mind the young age of the patient, the soft-walled vessel without calcium and the low radial strength required, it appeared to be an interesting option. The dissection progressed, requiring a new stent, as is often the case. It has been reported that about 25% of cases of successful PCI in SCAD require the placement of ≥2 further stents due to propagation of the dissection.17 We believe the traumatic dissection of the ostium is evidence of the frailty of the whole vessel intima, since no aggressive cannulation was performed.
The discrepancy in lumen diameter during coregistration is an intriguing feature in this case, which could easily be attributed to technical inaccuracy. In fact, a blood-filled false lumen gradually being filled with contrast is the most likely explanation for this finding. However, unlike blood, which has high backscatter and variable attenuation, a uniform and completely black structure on OCT must represent a transparent fluid-filled structure, which is usually contrast. OCT in this case shows a very large total diameter vessel (4.19 mm in Figure 3) and suggests the false lumen was filled not with blood, but with a clear fluid which angiography indicated was not contrast, since it corresponds to a small diameter vessel (with contrast only in the true lumen – red arrow in Figure 3). Because a false lumen may require several contrast injections until it is completely opacified, especially if it is long and with a small entry point, comparison of OCT and angiography pictures taken after consecutive injections may be misleading, since the contrast content of the false lumen may be different. The coregistration applied in this case is essential in this regard, since it gives real-time coordinated comparison of the images. To our knowledge, a vessel wall effusion with clear fluid has never been reported in the pathophysiology of SCAD, so the explanation for this finding remains debatable, as it is difficult to interpret.
ConclusionsSCAD is a differential diagnosis that should be borne in mind in cases of ACS, especially in young women without cardiovascular risk factors.
An initially conservative approach should always be considered in SCAD, and anticoagulation may not be of benefit in all cases.
The pathophysiology of SCAD is not fully understood. Effusion of fluid other than blood could play a part in its development prior to intimal tear or intramural hematoma.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.