Acute contrast-induced thrombocytopenia is a rare event with the use of modern low osmolarity iodinated contrast media. The pathophysiological mechanism that causes platelet counts to drop has not been identified, but an immunological mechanism is suspected due to cytotoxicity after previous exposure to contrast. We report the case of a 47-year-old male patient with acute severe thrombocytopenia due to iodinated contrast media exposure. His platelet count after the procedure with the highest amount of contrast was zero, which is the lowest reported platelet count to date. Percutaneous coronary revascularization under both intravascular ultrasound and gadolinium contrast guidance was performed without complications. The most feared complication after the use of gadolinium is nephrogenic systemic fibrosis, especially in patients on hemodialysis.
A trombocitopenia aguda induzida pela utilização endovascular de contraste iodado de baixa osmolaridade é um acontecimento raro com os novos contrastes. O mecanismo fisiopatológico que provoca a diminuição do número de plaquetas ainda não foi esclarecido, mas suspeita-se que possa ser devido a um mecanismo imunológico por toxicidade após a exposição ao contraste. Apresenta-se o caso clínico de um homem de 47 anos com trombocitopenia aguda severa, devido à exposição a contraste iodado. A contagem de plaquetas após o procedimento desceu para zero, o que corresponde ao valor mais baixo alguma vez reportado. A revascularização miocárdica percutânea foi realizada guiada por IVUS e gadolínio, sem complicações. Após a utilização do gadolínio, a complicação mais temível é a fibrose fefrológica aguda, especialmente em doentes em hemodiálise.
Iodinated contrast media, used in coronary angiography, can occasionally produce serious reactions including respiratory failure or anaphylactic shock. Acute contrast-induced thrombocytopenia is an unusual event with the use of modern low osmolarity iodinated contrast media. The mechanism behind contrast-induced thrombocytopenia remains unclear, but an immunoallergic or idiosyncratic reaction and direct toxicity have been postulated. Gadolinium is a contrast medium used as an alternative in cases of intolerance or contraindication to iodinated contrast. Here, we present a case of a male patient with acute severe thrombocytopenia induced by iodinated contrast media, who underwent percutaneous coronary revascularization under both intravascular ultrasound (IVUS) and gadolinium contrast guidance.
Case reportWe present the case of a 47-year-old man, hypertensive, an ex-smoker, who received a kidney transplant in 1993 and restarted hemodialysis in 1999 due to recurrent focal segmental glomerulonephritis. In 2007, the patient complained of typical chest pain. After pretreatment with 100 mg aspirin and a 300-mg loading dose of clopidogrel, coronary angiography was performed, which revealed no significant coronary lesions. At that time, severe thrombocytopenia was detected (7×109/l), which was attributed to the pretreatment with clopidogrel. The patient remained asymptomatic until 2014, when he presented angina symptoms. Exercise testing was positive and a new coronary angiography was performed via the right femoral artery, without clopidogrel but still using aspirin (the patient had previously taken aspirin, but it had to be discontinued due to an episode of epistaxis) and 2000 IU of unfractionated heparin as pretreatment. Multivessel disease (Figure 1A and D) was diagnosed. After 24 hours, the patient's platelet count dropped from 242×109/l to 75×109/l with a nadir of 25×109/l after 48 hours; aspirin was then suspended and pseudothrombocytopenia was ruled out. Tests for heparin-induced thrombocytopenia were negative, and blood parameters and clotting times were normal. Possible explanations were that the episode was related to aspirin or was a hematologic reaction to catheterization contrast.
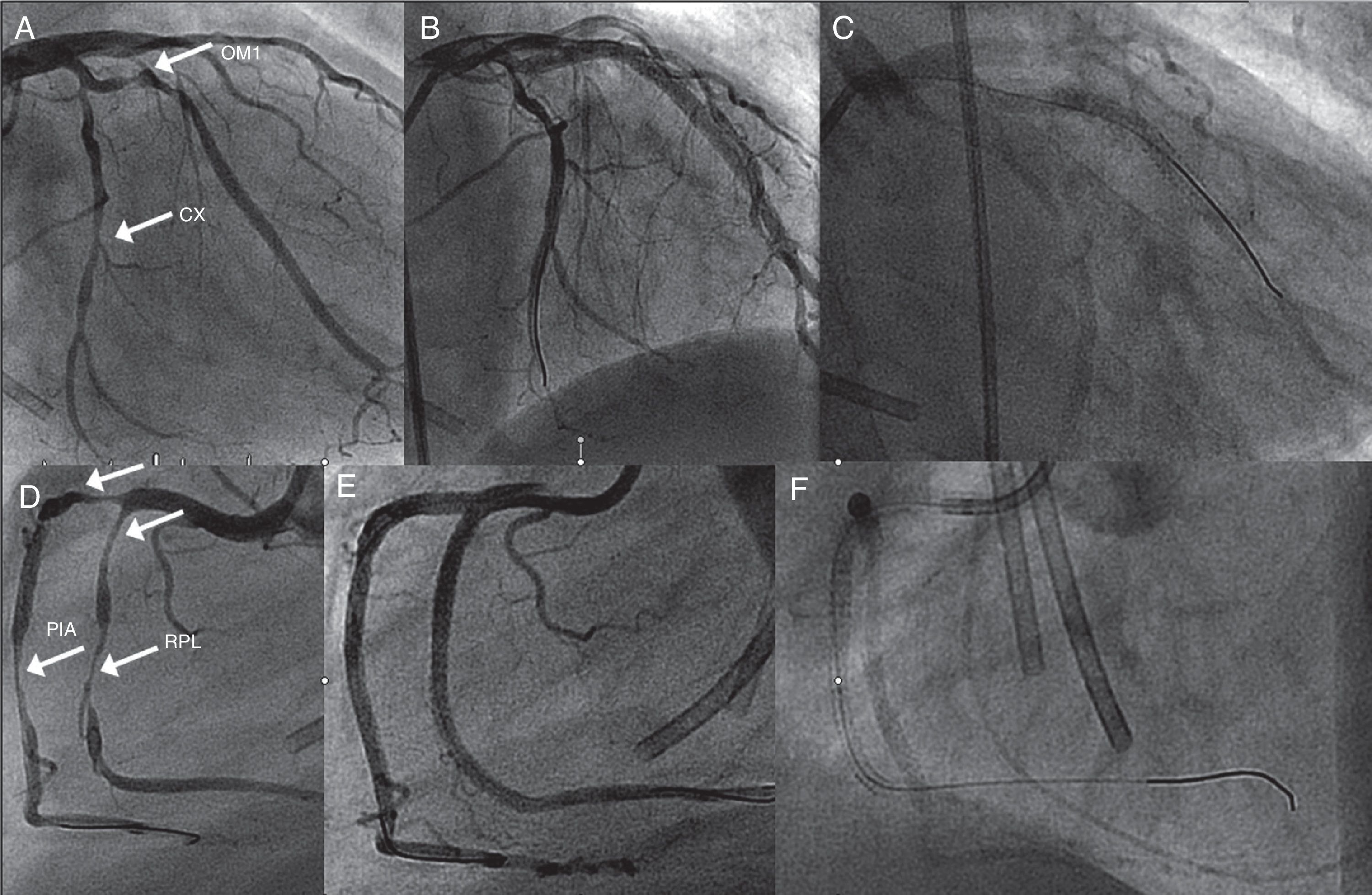
Coronary angiography showing (A) 85% long stenosis of the first obtuse marginal branch and 80% stenosis of the distal circumflex (CX) artery (arrows); (B) these lesions treated with two and one drug-eluting stents (DES), respectively; (C) the CX with gadolinium contrast after six months; (D) early right coronary artery (RCA) bifurcation, 90% stenosis of the right posterolateral branch (RPL) (arrows), 90% at the origin of the posterior interventricular artery (PIA) and 80% distal to the PIA; (E) these lesions treated with two DES in the RPL, one DES at the origin of the PIA and another in the distal segment of the PIA; (F) coronary angiography of the RCA with gadolinium contrast after six months. CX: circumflex artery; OM1: first obtuse marginal branch; PIA: posterior interventricular artery; RPL: right posterolateral branch.
The patient's platelet count returned to normal four days after the procedure. Prasugrel was then administered for three days without affecting the platelet count. Using 213 ml of ioxaglate sodium/ioxaglate meglumine ionic iodinated contrast (Hexabrix) and bivalirudin as an anticoagulant, percutaneous intervention was performed to implant seven zotarolimus-eluting stents (Figure 1B and E). After six hours, blood tests showed a platelet count of zero with no clinical manifestations; methylprednisolone was then administered gradually, and the platelet count recovered to 181×109/l after six days. The patient was diagnosed with severe thrombocytopenia after administration of iodinated contrast media.
A basophil activation test (CD69 expression on CD4+ lymphocytes) was negative for Hexabrix.
Six months after revascularization, the patient was readmitted with angina symptoms. He opted for conservative management and intensified treatment. However, he was readmitted three weeks later with refractory rest angina, and was referred for coronary angiography. After being informed of the risk of nephrogenic systemic fibrosis (NSF), the patient agreed to the procedure. Gadolinium (gadoteridol) was used as a contrast medium, using only 36 cc, and IVUS studies indicated severe restenosis of the previously implanted stents in the first obtuse marginal branch and the right coronary artery (Figures 1C and F and 2A and C). Coronary surgery was proposed and rejected by the heart team, so percutaneous revascularization was performed in two stages (Figure 2B and D), using 42 cc of gadoteridol in each procedure and hemodialysis four hours after the procedure to reduce the risk of NSF. Platelet counts were unchanged after these procedures, confirming the diagnosis of thrombocytopenia induced by iodinated contrast. No adverse events were reported on admission. After two years of follow-up, the patient has had no unstable angina pectoris and has not developed signs or symptoms suggestive of NSF.
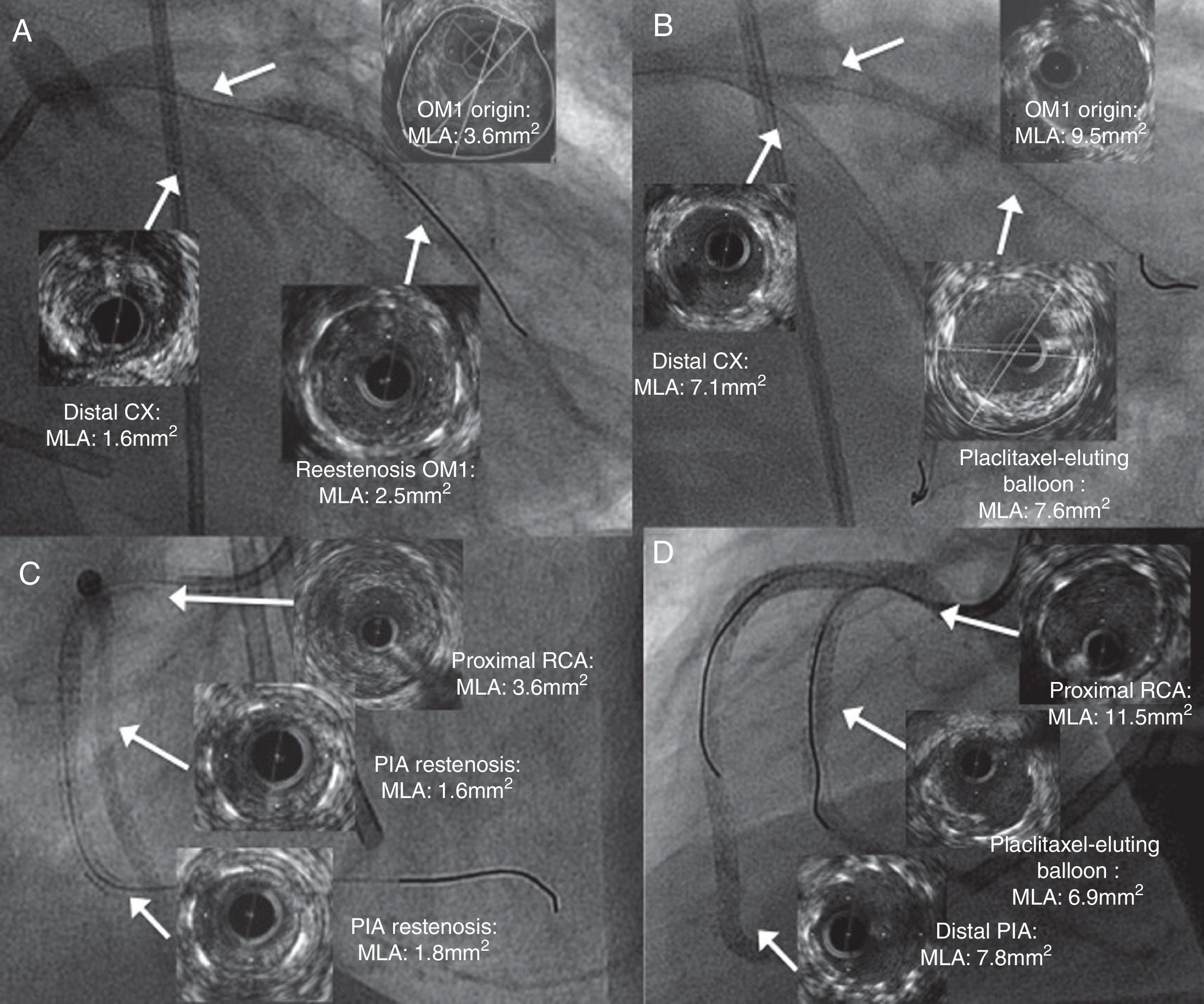
Coronary angiography with gadolinium contrast assessed by intravascular ultrasound showing significant stenosis (arrows): (A): in-stent restenosis (ISR) of the first obtuse marginal (OM1), subocclusive stenosis of the distal circumflex (CX) artery and at the origin of the OM1; (B) ISR treated with a paclitaxel-eluting balloon, and both stenoses treated by double-kissing crush stenting with two drug-eluting stents (DES) at the origin of the OM1 and CX; (C) ISR of the right posterolateral branch (RPL), of the distal edge of the distal stent of the PIA, and of the proximal stent segment of the posterior interventricular artery (PIA), affecting the proximal right coronary artery (RCA); (D) the RPL treated with a paclitaxel-eluting balloon, the distal PIA with one DES, and the proximal PIA of the RCA with a balloon and one DES, ending with double-kissing crush stenting from the PIA to the RPL. CX: circumflex artery; MLA: minimal lumen area; OM1: first obtuse marginal branch; PIA: posterior interventricular artery; RPL: right posterolateral branch.
Thrombocytopenia is one of the rarest complications associated with iodinated contrast, only 10 cases having been described in the literature.1–3 The pathophysiological mechanism that causes platelet counts to drop has not been identified, but an immunological mechanism is suspected due to cytotoxicity after previous exposure to contrast.1 Some authors have suggested the possibility of direct toxicity of the iodinated contrast medium2; in our case, immunoallergic tests of platelet aggregation, which study the direct effect of contrast on platelets, produced negative results, suggesting an idiosyncratic mechanism in a patient who showed hypersensitivity to iodinated contrast.4
Our patient remained asymptomatic and had no serious bleeding events, although his platelet count after the procedure with the highest amount of contrast was zero, which is the lowest reported platelet count to date. This complication is severe, but conservative management can be used in combination with avoidance of situations that encourage bleeding. Transfusion of platelet concentrates may not be necessary.
Gadolinium is a contrast with low radiopacity, which limits the proper assessment of angiographic lesions, so the medium is not routinely used for coronary angiography. Although a safe compound, gadolinium may cause renal failure5 in patients with creatinine levels higher than 3 mg/dl or if doses of more than 0.4 mmol/kg are used. The most feared complication is NSF, especially in chronic kidney disease (CKD) patients on dialysis. The incidence of NSF is below 0.5%, but the condition is potentially serious and requires a lengthy follow-up, as there can be a four-month delay before symptom onset,6 and it may occur up to three years after gadolinium exposure. For CKD patients, the amount of gadolinium administered should be controlled (never exceeding three times the recommended dose of 0.2 ml/kg),7 and hemodialysis should be performed as early as possible after coronary angiography. Percutaneous coronary revascularization with gadolinium contrast is possible in spite of its low radiopacity provided it is guided by IVUS, which enables the real size of the vessel to be measured and the result to be confirmed and optimized, leaving no residual coronary dissection or stent underexpansion. It also enables the use of low doses of gadolinium.
ConclusionsPercutaneous coronary revascularization may be carried out under both IVUS and gadolinium contrast guidance in patients with acute iodinated contrast-induced thrombocytopenia. After using gadolinium, the most feared complication is NSF, especially in patients on hemodialysis, so the amount of gadolinium administered must not exceed 0.2 ml/kg.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.