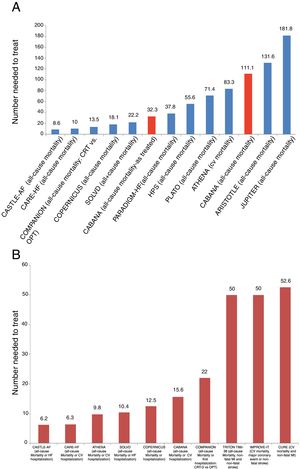
Despite mixed reactions among the cardiovascular community, the recently published Catheter ABlation vs ANtiarrhythmic Drug Therapy in Atrial Fibrillation (CABANA) trial should be considered positive, as it provides clear evidence that catheter ablation is a valuable option for treating patients with atrial fibrillation (AF). In CABANA, not only did catheter ablation reduce the AF burden and prolong time to first recurrence,1 but it also improved quality of life.2 Furthermore, and most importantly, it reduced all-cause mortality and cardiovascular hospitalizations (HR 0.83, 95% CI 0.74-0.93, p=0.001).1 What this means is that for every 15.6 patients randomized to catheter ablation, one death or cardiovascular hospitalization was prevented (number needed to treat [NNT] 15.6). This is an extremely informative and powerful endpoint, as it is used for showing the benefit of most interventions across the spectrum of cardiovascular disease (Figure 1). The fact that it is a secondary endpoint in CABANA does not reduce its importance.
Clinical events in some of the main cardiovascular trials. (A) and (B) show the number of patients who need to be treated for a fatal event to be prevented (A), or for a fatal event or hospitalization to be avoided (B). We chose to include trials of different cardiovascular interventions that have demonstrated significant benefit in the prevention of these events. Interpretation of these graphs shows that among all these interventions whose benefit has been demonstrated in landmark trials, and most of which have led to indications in the guidelines, the interventions with more benefit, as represented by a larger number of lives saved or hospitalizations avoided, are those with a smaller number needed to treat to prevent one event. As such, interventions with most benefit as measured by the number needed to treat are those which are located closer to the origin on the x axis. Furthermore, in (A) the results of CABANA are highlighted in red for two different reasons: first, inclusion of the results of the as-treated analysis, with an NNT of 32.3, and secondly, inclusion of the results of the ITT analysis, which were not significant due to the small sample size. However, CABANA shows a larger, albeit non-significant, impact than the novel oral anticoagulants and statins in some populations. Randomized trials for: AF – CASTLE-AF and CABANA; cardiac resynchronization therapy – CARE-HF and COMPANION; beta-blockers – COPERNICUS; angiotensin-converting enzyme inhibitors – SOLVD; PARADIGM-HF – valsartan + sacubitril vs. enalapril; statins and treatment of dyslipidemia – HPS, JUPITER and IMPROVE-IT; anti-platelet agents – CURE, PLATO and TIMI-38 TRITON; anti-arrhythmic agents – ATHENA; anticoagulants – ARISTOTLE. CV: cardiovascular; CRT: cardiac resynchronization therapy; CRT-D: cardiac resynchronization therapy-defibrillator; HF: heart failure; MI: myocardial infarction; OPT: optimal pharmacologic therapy.
The patient subgroup that derived most benefit from catheter ablation was younger individuals (less than 65 years old).1 This result is not surprising considering that most of these patients live long enough to experience a mortality benefit from the intervention.
In CABANA, no atrio-esophageal fistulas, stroke or procedure-related mortality were observed. Procedure-associated complications included myocardial infarction in <1/1000, need for pericardiocentesis in 0.8%, and vascular access complications in 3.9%. At the same time, ventricular tachycardia or ventricular fibrillation was observed in 0.8%, and thyroid dysfunction in 1.6%, in the medical treatment arm. This eases some of the concerns associated with ablation3 and illustrates that being assigned to antiarrhythmic agents is also not devoid of risk.
Issues with study design and arising during the trial1. Nearly a third of patients initially randomized to treatment with antiarrhythmic agents were later treated with ablation. On the other hand, almost 10% of patients randomized to catheter ablation did not receive this treatment. These are excessively high cross-over rates, and are unequal in the two treatment arms.
2. There were weaknesses in the selection and definition of the trial's primary endpoint. This was changed during the course of CABANA, from all-cause mortality to a combined endpoint of all-cause mortality, incapacitating stroke, major bleed or cardiac arrest. Why did the investigators not choose a more widely accepted endpoint, like all-cause mortality and hospitalizations? A recent trial comparing transcatheter aortic valve replacement and surgical aortic valve replacement in low surgical risk patients has been hailed as a success, as the newer treatment reduced the rate of the composite of death, stroke, or rehospitalization at one year.4 Would not the same apply to CABANA if it had used a similar primary endpoint? It should be recalled that the intention-to-treat (ITT) analysis showed that catheter ablation significantly reduced mortality and hospitalizations, and the rate of stroke in the catheter ablation arm was numerically 50% lower, which leads one to suspect that such an endpoint would also very likely show a significant difference.
It is interesting to note that the combined primary endpoint in CABANA included events like stroke and bleeding, as well as mortality and cardiac arrest. Ablation was numerically better for all of them except bleeding, for which the event rate was the same (as would be expected as patients in both treatment arms were on anticoagulants).
3. Patients who had previously not tolerated or had relapsed on more than two antiarrhythmic drugs, or were on full-dose amiodarone, were not included in the study. CABANA therefore excluded a good percentage of real-world AF patients currently being referred for ablation.
4. To be included in CABANA, patients had to be eligible for catheter ablation.5 However, some patients who were included in the study and randomized to ablation ended up not having the procedure. In the lead author's own words, “the main reason for that is that patients changed their minds, or a physician changed their mind for them. Or if you go to China or Korea, there are other conditions that are important there.” With regard to this point, the Medscape editor responsible for the article added: “In Packer's HRS presentation, he said of these countries that often ‘if you don’t go into the hospital with a bag of money, you will not get ablated.”’6 Box III in CABANA appears to confirm this by showing that patients randomized to the ablation arm but not receiving ablation were more frequently from ethnic minorities.1 This raises several questions regarding the eligibility of these patients and the trial's organization. If money was required for patients to have an ablation procedure and they were unable to pay, should they have been considered eligible for the trial in the first place? Should centers that behaved in this way toward clinical trial patients have been allowed in the trial? In mega-drug trials, the sponsor provides the drug free of charge. Could CABANA not have done the same with the ablation treatment for these patients?
Benefit of catheter ablation for heart failure patientsCABANA included AF patients with and without heart failure. The ITT sub-analysis of the primary endpoint for heart failure patients suggests a benefit of ablation, with a 39% risk reduction, which nearly crosses the boundary of significance. This was despite there being only 1197 patients in the study with heart failure.1
The CASTLE-AF trial suggested a clear benefit of this treatment modality in the subgroup of patients with AF and heart failure and left ventricular dysfunction.7 The NNT to save a life was 8.6, and to prevent one heart failure hospitalization it was 6.6. These results are therefore in line with the trend observed in CABANA, and with recently published systematic reviews of randomized trials that showed a similar reduction in mortality and effect size with catheter ablation in the AF population. This effect became more pronounced in sub-analyses of studies including only heart failure patients.8,9
Several factors have been suggested to explain the benefit of AF ablation in patients with heart failure, which are discussed in more detail by Kadhim et al.10 Statistical considerations concerning this subject are discussed in Box II.
Effect size of catheter ablation on hard outcomesFigure 1 shows the effect size, represented by the NNT, of several drugs and interventions currently used in cardiovascular medicine and recommended in international guidelines. It is interesting to note that the effect size and benefit for some of these drugs appear to be much lower than that observed with catheter ablation (Figure 1A and B). Using apixaban, one of the novel oral anticoagulants, instead of a vitamin K antagonist, we would need to treat 132 AF patients to save one life,11 while in the population with heart failure in PARADIGM-HF, the association of valsartan and sacubitril needs to be used instead of enalapril in 37.8 and 35.7 patients to save one life or prevent one heart failure hospitalization, respectively.12 This is also seen with drugs whose use is considered unquestionable in coronary artery disease, like ticagrelor instead of clopidogrel, for which the number of patients needed to treat to prevent one fatal event is 71.4.13
Even the most conservative analysis of CABANA (the ITT analysis “shows a clear significant reduction”) of the all-cause mortality and cardiovascular hospitalization endpoint (HR 0.83, 95% CI 0.74-0.93, p=0.001), which translates into an NNT of 15.6.1
The question of ablation timing and level of evidence in the guidelinesAre the data sufficient to support a strategy of early AF ablation – the earlier the better? Knowledge of predictors of success for this procedure, like AF episode duration and left atrial size, and the fact that ‘AF begets AF’ and that younger patients derive more benefit, appear increasingly to support such a strategy.14,15
As a matter of coherence, it cannot be much longer before the next AF guideline updates include a clear recommendation for this procedure with the aim of improving survival in patients with heart failure and LV systolic dysfunction (class of recommendation I, level of evidence A), and expand the current class I, level of evidence A indication offering catheter ablation to patients with paroxysmal AF and recurrent AF while on antiarrhythmic agents, to include patients with persistent AF (as these represent the majority of patients in the CABANA and CASTLE-AF trials). Table 1 provides some information on the available evidence and how this is incorporated in the current European and American classification of level of evidence. At this point, the need for a randomized controlled trial comparing catheter ablation and a sham procedure, as previously conducted for percutaneous coronary intervention in stable angina,16 is highly debatable.
Levels of evidence for the impact of atrial fibrillation rhythm control strategies on mortality and hospitalizations.
| Catheter ablation | Antiarrhythmic drugs |
|---|---|
| Level of evidence A Data derived from multiple RCTs or meta-analyses | |
| Mortality | Mortality |
| RCTs | RCTs |
| CASTLE-AF7a 46%b | PALLAS18 111%b dronedarone |
| AATAC17a 56%b | Meta-analyses |
| Meta-analyses | Lafuente-Lafuente et al.19 |
| Barra et al.8 55%b | 123%b sotalol |
| Turagam et al.9a 48%b | 139%b quinidine and disopyramide |
| Freemantle et al.20 | |
| 173%b amiodaroneb | |
| 332%b sotalol b | |
| Hospitalizations | |
| RCTs | |
| CASTLE-AF7a 50%b | |
| AATAC17a 45%b | |
| Meta-analyses | |
| Turagam et al.9a 40%b | |
| Mortality and hospitalizations | |
| RCTs | |
| CABANA1 17%b | |
| CASTLE-AF7a 38%b | |
| Level of evidence B Data derived from a single RCT or large non-randomized studies | |
| Mortality | |
| Large non-RCTs | |
| Friberg et al.21 50%b | |
| Hospitalizations | |
| RCTs | |
| PALLAS18 81%b Dronedarone | |
| ATHENA22 26%b Dronedarone | |
| Mortality and hospitalizations | |
| RCTs | |
| ATHENA22 24%b Dronedarone | |
The main messages of this positive study, which confirms the benefit of catheter ablation of AF on hard outcomes, are highlighted in Box III.
Box I. The CABANA trial: types of analysis and impact on hard outcomes
- a)
Intention-to-treat (ITT): This analysis compares patients based exclusively on the treatment assigned at randomization. This is the method preferred by methodologists and was the planned analysis for CABANA right from the start. However, it is important to note that 102 patients (9.2%) randomized for ablation in CABANA did not receive this treatment, and 301 patients (27.5%) randomized for medical therapy ended up receiving ablation, but in the ITT analysis they appear in the ablation and medical therapy arms, respectively. Interestingly, ITT analyses are discouraged when adherence rates differ between groups,23 which appears to have been the case in CABANA. ITT analyses are usually considered to be more conservative (except for situations when the reference treatment is more effective than the new treatment, which is clearly not the case with ablation). Also, ITT analyses provide information on the average causal effect of being assigned a treatment. A different type of analysis should be adopted if one wants to know the actual average causal effect of receiving a treatment.23 In our view, the ITT analysis shows what happens when planned ablation at randomization is compared to medical therapy and possible ablation at a later date.
↓ All-cause mortality and cardiovascular hospitalization: HR 0.83, 95% CI 0.74-0.93, p=0.001, NNT 15.6
- b)
As-treated (treatment received): This analysis compares patients who actually received ablation with those who were treated with antiarrhythmic agents only. The concern with this analysis is that it may remove the effect of randomizing of patients into two equal groups.
↓ All-cause mortality: HR 0.60, 95% CI 0.42-0.86; p=0.005, NNT 32.3
↓ All-cause mortality and cardiovascular hospitalization: HR 0.83, 95% CI, 0.74-0.94; p=0.002, NNT 3.0
↓ Combined primary endpoint: HR 0.67, 95% CI 0.50-0.89, p=0.006
- c)
Per-protocol: This analysis censors patients who cross over to a different treatment from that assigned at randomization. In the drug treatment group, follow-up of patients who received drug therapy and crossed over to catheter ablation was censored at the time of ablation. The per-protocol catheter ablation group included patients randomized to catheter ablation who received ablation within a pre-specified time window. Results for patients who received ablation in the first three months are shown below (similar results were observed for those receiving ablation within six and 12 months; however, it should be borne in mind that some patients were randomized to ablation and had to wait for months before having the procedure, and during that period (sometimes over six months) they were already in the ablation arm of the study).
↓ All-cause mortality: HR 0.68, 95% CI 0.47-0.99, p=0.047
↓ Combined primary endpoint: HR 0.73, 95% CI 0.54-0.99, p=0.046
Note: The NNT could only be estimated when the percentage of events was available.
Box II. Catheter ablation trials: considerations of effect size, power and sample size
It has been clearly demonstrated that to show a similar reduction in relative risk (e.g. 30%) in two populations with different baseline absolute risks (e.g. 1% in population A and 20% in population B), the population with lower risk at baseline would need a much larger sample (29 396 patients for the population with 1% risk at baseline vs. 1228 patients for the population with 20% risk at baseline, assuming a 1:1 randomization, and α of 0.05 and β of 0.8). Accordingly, it is not surprising that a clear benefit of catheter ablation was shown for patients with heart failure in CABANA, given their higher baseline risk for hospitalization or all-cause mortality (and thus requiring a smaller sample).
On the other hand, showing a significant reduction in stroke may be a more difficult task. In a world where, unlike in AFFIRM24 or RACE,25 nearly all AF patients are anticoagulated, the incidence of stroke is now very low (about 1.25% annually in the ENGAGE AF-TIMI 48 trial among patients treated with vitamin K antagonists or edoxaban 60 mg daily).26 Using the figures from the previous example (starting from a baseline absolute risk of 1.25%, and aiming for a 30% relative risk reduction, with 1:1 randomization and α=0.05 and β=0.8), it would be necessary for a randomized trial to include at least 23 466 participants to show such a protective effect. This study population would be about 10 times the size of CABANA (which included only 2204 patients).
Besides, as AF patients have multiple other cardiovascular risk factors (including hypertension, diabetes, obesity, sleep apnea, etc), the treatment of an atrial myopathy will not by itself protect them from all the associated stroke and cardiovascular risk factors.
Box III. CABANA trial: summary, assessment and take-home messages
- 1.
Patients who received ablation had lower mortality and cardiovascular hospitalization rates.
- 2.
Mortality reduction with ablation may be observed in higher risk patients (heart failure population).
- 3.
The results were positive for younger patients, who are likely to live long enough to suffer the deleterious effects of AF.
- 4.
The severity of complications and side effects of ablation was comparable to the severity of antiarrhythmic complications in the medical treatment arm.
- 5.
Ablation reduced the total AF burden and prolonged the time to first AF relapse.
- 6.
Peculiar study design:
- a.
unusual and surprising choice of combined endpoint, which was used for the first (and likely only) time in this trial;
- b.
slow patient recruitment due to unusual inclusion criteria;
- c.
protracted study even after modification of the primary endpoint when the study was ongoing; this occurred due to the lower than expected number of events, and issues with estimation of required sample size.
- 7.
The main study findings were not novel and confirmed what we already knew.
The authors have no conflicts of interest to declare.