Management of patients with congenital heart defects and associated pulmonary arterial hypertension remains a major concern. With evolving targeted drug therapies and new iterations of transcatheter devices, treatment of appropriately selected patients with severe pulmonary hypertension, classically considered inoperable, has become feasible. We report the case of a patient with concomitant ruptured right sinus of Valsalva aneurysm and ventricular septal defect, with early reversal of suprasystemic pulmonary pressures following successful percutaneous closure of ruptured sinus of Valsalva.
O tratamento de pacientes com defeitos cardíacos congénitos e hipertensão pulmonar associada continua a ser um importante motivo de preocupação. Com o desenvolvimento de estratégias farmacológicas dirigidas e novos dispositivos transcatéter, o tratamento de pacientes selecionados com hipertensão pulmonar grave, tradicionalmente considerados inoperáveis, tornou-se viável. Apresentamos o caso de um paciente com aneurisma do seio de Valsalva direito roto e defeito do septo ventricular, com rápida reversão das pressões pulmonares suprassistêmicas após encerramento percutâneo do seio de Valsalva roto.
Sinus of Valsalva aneurysm (SVA) is a rare condition affecting 3% of Asian and <1% of Western patients undergoing cardiac surgery, with a 4:1 male predominance.1 Etiology is either congenital or acquired secondary to infective endocarditis, syphilis, atherosclerosis, Marfan syndrome, autoimmune diseases or chest trauma. Aneurysms arising from the right sinus are the most common, usually rupturing into the right ventricle. Nearly 50% of SVAs coexist with a long-standing primary ventricular septal defect (VSD), most often supracristal. Rupture typically occurs in the mid-thirties, with variable severity of symptom onset according to size, location, associated lesions and acuity of the rupture. If left untreated, right ventricular overload and pulmonary arterial hypertension (PAH) develop, with a grim prognosis (one-year life expectancy).2 While surgery remains the mainstay treatment for ruptured SVA, percutaneous treatment has emerged as a valid alternative for selected patients.3
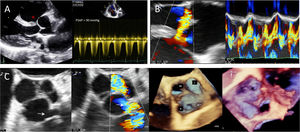
Case reportA 39-year-old man presented with a two-month history of worsening dyspnea and rapidly progressive orthopnea, in New York Heart Association (NYHA) functional class III. Physical examination revealed a continuous systolic-diastolic murmur in the pulmonary area and raised jugular venous pressure, but no cyanosis. The electrocardiogram showed sinus tachycardia with right bundle branch block, and the chest X-ray revealed cardiomegaly with pulmonary venous congestion. Transthoracic echocardiography (TTE) revealed right-sided volume overload with right ventricular enlargement (45 mm), dilated pulmonary trunk (38 mm) with near-systemic PAH (>90 mmHg), and the presence of a perimembranous ventricular septal defect (VSD), with normal left ventricular size and function (Figure 1A). Transesophageal echocardiography (TEE) confirmed the presence of a 3-mm perimembranous VSD with bidirectional shunting (predominantly right-to-left) (Figure 1B), while also revealing a 7-mm right SVA ruptured into the right ventricular outflow tract adjacent to the pulmonary valve (Figure 1C). Following multidisciplinary heart team discussion and considering the anatomical suitability of the rupture opening for a transcatheter approach (moderate sized defect, optimal proximal and distal margins, no associated aortic regurgitation), the patient was referred for transcatheter ruptured SVA closure, after prior balloon test occlusion to assess PAH reversibility.
(A) Transthoracic echocardiography showing a perimembranous ventricular septal defect (VSD, asterisk) with severe pulmonary hypertension; (B) two- and three-dimensional transesophageal echocardiography confirming VSD with bidirectional shunting, and unmasking a circular rupture opening of the right sinus of Valsalva aneurysm (6.5 mm×6.7mm, arrow) into the right ventricular outflow tract (C).
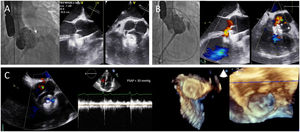
The procedure was performed under fluoroscopic and TEE guidance, using a retrograde approach, under intravenous heparin (80 U/kg) and antibiotic cover (cefazolin). A Swan-Ganz thermodilution catheter was inserted through the right cephalic vein. Baseline invasively determined pressures showed suprasystemic PAH with a mean pulmonary pressure of 59 mmHg and a mean aortic pressure of 50 mmHg. A pigtail catheter for aortography was advanced from the left femoral artery. Through right femoral arterial access, the SVA defect was crossed retrogradely from the aorta into the right ventricular outflow tract with a Judkins right catheter (Cordis Corp., Miami, FL), which was exchanged for a Mullins sheath (Cook Inc., Bloomington, IN) through a stiff exchange wire. A 10/5-mm Amplatzer Vascular Plug III (Abbott Vascular, Santa Clara, CA) was first advanced through the Mullins sheath to occlude the ruptured SVA for 30 min with incomplete shunt sealing, and thereafter replaced by a 12-mm Amplatzer Muscular VSD device, resulting in successful shunt occlusion (Figure 2A). Rapid hemodynamic improvement was seen during the transient occlusion test, with a marked reduction in mean pulmonary pressure (from 59 to 49 mmHg), increase in mean aortic pressure (from 50 to 73 mmHg), and normalization of oxygen saturation (SpO2 at 4 l/min from 86% to 100%) (Table 1). After confirmation of the suitability for closure of the ruptured SVA, the 12-mm Amplatzer Muscular VSD device was released, with minimal residual peri-device leak, no aortic regurgitation and inversion of the VSD shunt (Figure 2B). The small VSD was considered hemodynamically insignificant (restrictive) and was therefore untreated. The patient was discharged with no complications on dual antiplatelet therapy for six months, and under sildenafil 20 mg/8 h and furosemide 20 mg/24 h. At one-year follow-up, the patient remained without events, in NYHA functional class I, with minimal resting left-to-right shunt, and complete regression of suprasystemic PAH, allowing discontinuation of furosemide and sildenafil (Figure 2C).
Fluoroscopic and echocardiographic images of the ruptured sinus of Valsalva aneurysm (arrow) during transient percutaneous occlusion testing (A) and after definitive closure (B) with the 12-mm Amplatzer Muscular VSD device, with trace peri-device leak and trivial left-to-right shunt through the VSD; (C) one-year echocardiographic follow-up showing appropriate apposition and sealing of the device and absence of pulmonary hypertension.
The present case demonstrates the reversal of severe flow-mediated PAH related to systemic-to-pulmonary shunting with pulmonary overcirculation following transcatheter closure of a ruptured SVA into the right ventricle.
SVA are generally silent until rupture into a cardiac chamber. In our patient with concomitant SVA and VSD, the right coronary cusp aneurysm probably occluded the VSD until the sinus ruptured into the right ventricle, resulting in a large systemic-to-pulmonary shunt through the ruptured SVA and causing systemic systolic pressure overload in the pulmonary artery and systemic diastolic pressure and volume overload in the right ventricle, with predominant right-to-left shunting through the VSD and desaturation. Interestingly, closure of the ruptured SVA led to resolution of the aortocardiac shunt, with inversion of the VSD shunt to left-to-right shunting and normalization of saturation.
The degree of reversibility of the pulmonary circulation can be assessed using short-acting pulmonary vasodilators, or through transient balloon test occlusion, particularly in patients with persistently elevated pulmonary vascular resistance despite a positive response to vasodilators, and those with bidirectional shunting.4 Although somewhat arbitrary, a drop in pulmonary pressures of ∼20% without a significant decrease in systemic pressure during test occlusion would suggest a high likelihood of benefiting from permanent closure. Closure of cardiac shunts is generally not recommended when pulmonary artery pressures are >2/3 of systemic values,5 due to increased risk of sustained PAH, right heart failure and hypertensive crisis immediately after closure. However, the rationale for closure in our patient was the subacute onset of symptoms leading to rapid clinical deterioration and the presence of bidirectional shunting through the VSD, along with marked hemodynamic improvement after the transient occlusion test, making the hypothesis of fixed (irreversible) PAH highly unlikely, and thus highlighting a potential benefit from permanent shunt closure.
In recent years, percutaneous closure of ruptured SVAs has emerged as a valid alternative to surgery. Although direct comparisons between the two approaches have been somewhat limited by selection bias (more severe aortic regurgitation and more complex associated lesions in patients undergoing surgery), transcatheter closure of ruptured SVAs has been shown to be safe and effective.3 Suitability for percutaneous closure of intracardiac communications with associated PAH relies on anatomic features – magnitude of the shunt, presence of adequate rim to secure the device – and potential for PAH reversal. In our patient, three-dimensional TEE was crucial to precisely delineate two closely adjacent congenital defects, the shunt flows of which overlapped on TTE, showing anatomical suitability for percutaneous closure of the ruptured SVA. Although lacking solid scientific evidence, transcatheter closure of a ruptured SVA might be a reasonable option for patients with a rupture opening of less than 9 mm,6 in the absence of secondary complications (fistulous ruptured SVA or multiple rupture sites, significant aortic regurgitation) or pre-existing conditions requiring surgical correction (endocarditis, large VSD or other complex congenital defects).3 Of note, large lesions (>12 mm) should prompt preprocedural multimodality imaging assessment (including computed tomography or magnetic resonance imaging) to elucidate the best therapeutic approach.3 Most importantly, while transcatheter occlusion of ruptured SVAs has emerged as a well-established alternative to surgical closure for highly selected patients, caution is warranted in the setting of associated severe PAH. Transient occlusion testing during cardiac catheterization is critical in determining the safety of permanent shunt closure, enabling differentiation between dynamic PAH and irreversible obstructive PAH, and providing reliable assessment of the reversibility of pulmonary vascular disease. To our knowledge, this is the first case report of suprasystemic flow-induced PAH regression following percutaneous closure of a ruptured SVA.
Conflicts of interestThe authors have no conflicts of interest to declare.