Oleuropein is a polyphenol found in olive trees that has shown beneficial effects in animal studies and potentially in human health, although few studies have been performed to confirm this hypothesis in the latter population. Previous studies related its antioxidant activity to cardioprotective effects and showed a positive correlation between dose and response. We thus aimed to assess the cardioprotective effect of oleuropein and olive leaf extract in animal experiments. A literature search was conducted in June 2020 in the PubMed, Scopus and Web of Science databases. The descriptors “oleuropein” and “oleuropein aglycone” identified 12 articles for qualitative synthesis. Risk of bias was assessed by SYRCLE's RoB tool for animal studies. The results demonstrate evidence of a positive association between the administration of oleuropein and olive leaf extract and improvement in outcomes in hypertension, heart failure, myocardial infarction. renal hypertension and diabetes. This review presents a positive effect of oleuropein and olive leaf extract administration on cardiovascular parameters in animal studies.
A oleuropeína é um polifenol encontrado nas oliveiras que tem efeitos benéficos em estudos com animais e potencialmente na saúde humana, embora poucos estudos tenham sido realizados para justificar essa hipótese. Em estudos prévios a atividade antioxidante foi relacionada aos efeitos cardioprotetores e mostraram uma correlação positiva entre a administração e a resposta. Portanto, o objetivo do estudo foi avaliar o efeito cardioprotetor da oleuropeína e da folha de oliveira em experimentação animal. A pesquisa preliminar foi realizada em junho de 2020 nas bases de dados Pubmed, Scopus e Web of Science. Os descritores oleuropein e oleuropein aglycone resultaram em 12 artigos para síntese qualitativa. O risco de viés foi avaliado pela ferramenta SYRCLE ROB adaptada para estudos em animais. Os resultados demonstram evidências de associação positiva entre a administração de oleuropeína e folha de oliveira em desfechos relacionados na melhoria da hipertensão arterial, insuficiência cardíaca, enfarte agudo do miocárdio, hipertensão renal e diabetes. Esta revisão apresenta uma associação positiva da administração de oleuropeína e folha de oliveira acerca dos parâmetros cardiovasculares em estudos com animais.
The branches and leaves of the olive tree (Olea europaea L.) are rich in polyphenols that confer benefits to human health.1 Interest in olive production around the world has focused on the recognized benefits of regular consumption of extra virgin olive oil.2–4
The main polyphenols present in olive leaves are oleuropein and hydroxytyrosol, and olive leaf extract (OLE) has been used in some countries.5 The use of leaves is sustainable since they are normally discarded during the production of olive oil, as well as being due to the benefits of OLE itself.6 Oleuropein has shown cardioprotective, antioxidant, anti-inflammatory and anticancer effects.7
Although studies have assessed the effects of oleuropein consumption, few experimental studies have analyzed the systemic effect of the use of OLE or of the isolated compound itself. Analysis of in vivo outcomes helps describe how oleuropein behaves but does not provide a wider view of how it acts in the organism.
The use of OLE presents a potentially viable and sustainable health impact through food consumption.1,5
According to data from the World Health Organization, 17.9 million people die annually from cardiovascular disease, which represents 31% of all deaths. Various risk factors have been associated with cardiovascular disease, including hypertension, type 2 diabetes, dyslipidemia and obesity.8,9
The possible cardioprotective effect of oleuropein has mostly been studied by animal experimentation, with few data in humans.7 The aim of this study was to perform a systematic review of studies assessing the cardioprotective effect of oleuropein in animal experiments.
MethodsThis review is registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42019129083).
Search strategy and eligibility criteriaA search was conducted in June 2020 with the terms “oleuropein” and “oleuropein aglycone” in the PubMed, Scopus and Web of Science databases. The descriptors were based on the most sensitive model, aiming to capture all articles that fulfilled the inclusion and exclusion criteria. Restrictions regarding language, publication date or any other filter were not applied.
This systematic review included articles that (1) had as the experimental group rats or mice of any strain; (2) used oleuropein from OLE or the isolated compound; (3) used oral administration by oral gavage or dietary supplement; and (4) investigated cardiovascular outcomes related to diet, including hypertension, heart failure, high-calorie and high-fat diet, myocardial infarction, renal hypertension and diabetes.
Study selectionStudy selection took place in three stages: removal of duplicates and triplicates; screening; and full reading. A spreadsheet was designed in Microsoft Excel to perform the study selection. Independent review was carried out by the authors Menezes, Rafaella (M.R.); Peres, Kathleen (P.K.); Vale, Marina (V.M.); and Faccioli, Larissa (F.L.). After removal of duplicates and triplicates, two independent reviewers (M.R. and P.K.) analyzed titles and abstracts to sort potentially eligible articles. In the third stage, the same reviewers carried out a full reading of the studies. All disagreements were resolved by a third reviewer (V.M.).
Data extractionData extraction was performed independently by four authors (M.R; P.K; V.M; F.L.). A spreadsheet was purpose-designed in Microsoft Excel and frequent meetings were held to maintain the standard of analysis. The data extracted from the studies were article title, reference, population, intervention (type and follow-up time), control, outcome, limitations, and conclusions.
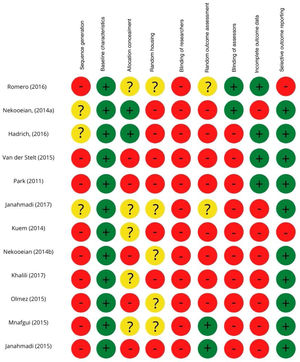
Assessment of risk of biasThe risk of bias was assessed using SYRCLE's RoB tool for animal studies and applied by four authors (M.R; P.K; V.M; F.L.). The following domains were considered: sequence generation; baseline characteristics; allocation concealment; random housing; blinding of researchers; random outcome assessment; blinding of assessors; incomplete outcome data; and selective outcome reporting.
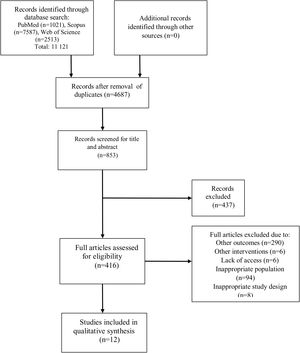
ResultsStudy selectionThe electronic search identified 11121 articles, of which 4687 were selected after exclusion of duplicates and triplicates. This selection was assessed by two reviewers (M.R. and P.K), resulting in 853 studies for full reading, after which 437 were excluded for the following reasons: assessment of other outcomes (n=290); inappropriate population (n=94), study design (n=8) or intervention (n=6); or lack of full article access (n=6). Following this assessment, 12 articles that investigated the effect of oleuropein and/or OLE in rodents in terms of cardiovascular outcomes were included in the qualitative synthesis, using the content analysis method after data tabulation (Figure 1).
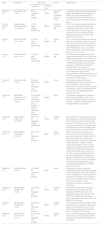
Study characteristicsOf the 12 articles selected, 11 used the isolated oleuropein compound as intervention and one used OLE. Exposure time ranged from seven days to 12 weeks. Detailed information on the studies is presented in Table 1.
Experimental studies assessing the cardioprotective effects of oleuropein and olive leaf extract.
| Study | Population | Intervention | Control | Main findings | |
|---|---|---|---|---|---|
| Type/dose | Exposure time | ||||
| Hadrich et al.10 | Male Wistar rats (n=50) | OL 50 mg/kg/day (oral gavage) | 8 weeks | Standard diet vs. cholesterol-rich diet (HCD) | OL treatment significantly decreased body weight, white adipose tissue weight, and plasma TG, total cholesterol and LDL cholesterol, as well as liver steatosis, in the HCD+OL group compared to the HCD group. |
| Van der Stelt et al.11 | Male wild-type C57BL/6JOlaHsd mice (n=36) | OL 758 mg/kg (dietary supplement) | 12 weeks | Standard normal-fat diet | The HFD+OL group showed significantly lower body weight and blood glucose compared to mice fed the control HFD. Serum leptin protein levels and gene expression levels in epididymal white adipose tissue of HFD+OL mice were lower than in HFD mice. |
| Park et al.12 | Male C57BL/6N mice (n=24) | OL 0.03% (w/w) (dietary supplement) | 10 weeks | Normal diet | OL in the HFD group significantly reduced absolute liver weight compared to the non-OLE HFD group and also reversed HFD-induced upregulation of the expression of LXR, PPARc2, LPL, aP2, Cyc-D, E2F1, CTSS, SFRP5 and a-SMA and collagen genes in the liver of mice. |
| Kuem et al.13 | Male C57BL/6N mice (n=24) | OL 0.03% (w/w) (dietary supplement) | 10 weeks | Normal diet | Cumulative body weight gain and total visceral fatpad weight in the OL group was significantly lower than in the HFD group. The OL group had decreased expression of SFRP2 and dickkopf 2 and increased expression of WNT10b in epididymal adipose tissue. mRNA levels of transcription factors (PPARγ and C/EBP α) and adipogenic target genes (lipoprotein lipase, fatty acid synthase, and aP2) were downregulated in the OL group compared to the HFD group. |
| Olmez et al.14 | Male Wistar rats (n=40) | Ethanolic OLE 20, 50, 100 mg/kg/day (oral gavage) | 8 weeks | Saline | OLE supplementation significantly decreased serum total cholesterol and LDL cholesterol in HCD rats. Rats receiving HCD showed a marked increase in atherogenic index, while treatment with OLE (50 and 100 mg/kg) significantly reduced this index. |
| Romero et al.16 | Male Wistar Kyoto rats (n=10) and male SHR (n=20) | OL-enriched (15% w/w commercial OLE). SHR group treated with OLE 30 mg/kg/day (oral gavage) | 5 weeks | Water | OLE exerted antihypertensive effects on SHR by reducing SBP and HR. It also improved endothelial function, prevented increase in NADPH oxidase activity and reduced MAPK phosphorylation. |
| Janahmadi et al.18 | Male Sprague Dawley rats (n=24-32) | OL 5, 10 or 20 mg/kg/day (dietary supplement) | 5 weeks | Distilled water | SOD and GRx in groups receiving 10 and 20 mg/kg/day OL were significantly higher than in the group receiving vehicle only. Serum concentrations of IL-1β and TNF-α in the groups receiving 10 mg/kg/day and 20 mg/kg/day OL were significantly lower than in the group receiving vehicle only. |
| Janahmadi et al.22 | Male Sprague Dawley rats (n=30-40) | OL 10, 20 or 30 mg/kg/day (oral gavage) | 7 days | Sham, vehicle | Stroke volume, ejection fraction, and cardiac output in the 20 mg/kg/day OL group were significantly higher than in the MI vehicle only group. Left ventricular internal diameter in systole, left ventricular internal diameter in diastole, systolic volume, and diastolic volume in the 30 mg/kg/day OL group were significantly lower than in the MI vehicle only group. Creatine kinase-MB, LDH, troponin I, IL-1β, TNF-α, and malondialdehyde in the 20 and 30 mg/kg/day OL groups were significantly lower than in the MI vehicle only group. SOD and GRx concentrations in the 20 and 30 mg/kg/day OL groups were significantly higher than in the MI vehicle only group. |
| Mnafgui et al.23 | Male Wistar rats (n=32) | OL 20 and 40 mg/kg/day (oral gavage) | 7 days | Saline | Isoproterenol-induced myocardial infarcted rats showed significant increases in HWI compared to control rats. However, 20 and 40 mg/kg/day OL decreased HWI compared to untreated rats. The effect of OLE on serum cardiac markers (CK-MB, ALT, LDH and troponin T) was significantly increased compared to normal rats. |
| Nekooeian et al.25 | Male Sprague Dawley rats (n=35) | OL 20, 40 or 60 mg/kg/day (oral gavage) | 4 weeks | Distilled water | Compared with the control group, the group with type 2 diabetes and renal hypertension (DM2+HTN-Veh) had significantly higher SBP, HR, and FBG. SBP, HR, and FBG in the OL-treated groups were significantly lower than in the DM2+HTN-Veh group. |
| Khalili et al.26 | Male Sprague Dawley rats (n=64) | OL 20, 40 or 60 mg/kg/day (oral gavage) | 4 weeks | Distilled water | OL reversed unfavorable changes in FBG, glucose tolerance, and serum lipid profile. Serum LDL cholesterol levels in the OL-treated groups were significantly lower than in the DM2+HTN-Veh group. |
| Nekooeian et al.27 | Male Sprague Dawley rats (n=37) | OL 20, 40 or 60 mg/kg/day (oral gavage) | 4 weeks | Distilled water | OL (20, 40 and 60 mg/kg/day) decreased blood pressure, blood glucose and FBG significantly compared to the DM2+HTN-Veh group. |
ALT: alanine aminotransferase; DM2+HTN-Veh: type 2 diabetes and renal hypertension treated with vehicle only; FBG: fasting blood glucose; GRx: glutathione reductase; HCD: high cholesterol diet; HFD: high fat diet; HR: heart rate; HWI: heart weight index; IL-1β: interleukin-1 beta; LDH: lactate dehydrogenase; LDL: low-density lipoprotein; MI: myocardial infarction; OL: oleuropein; OLE: oleuropein; SHR: spontaneously hypertensive rats; SBP: systolic blood pressure; SOD: superoxide dismutase; TG: triglycerides; TNF-α: tumor necrosis factor alpha; w/w: weight for weight.
SYRCLE's RoB tool was used to assess the risk of bias. Studies with a low risk of bias are marked with a positive sign and those with a high risk of bias with a negative sign (Figure 2). All selected studies had a low risk of bias for baseline characteristics between groups and most for selective outcome reporting. However, none of the selected studies reported methods of sequence generation or blinding of researchers and few reported having controlled for allocation concealment, random housing, random outcome assessment, blinding of assessors, or incomplete outcome data. The main variables that could significantly compromise the quality of this type of study were adequately controlled, and those that were less well controlled have less impact in animal studies. Therefore, the authors consider that the quality of the selected studies was not significantly compromised, and that the risk of bias was low.
DiscussionThe selected studies used different types of in vivo tests on rats and mice assessing the possible effects of OLE and oleuropein on the cardiovascular system, including hypertension, heart failure, high-fat diet, myocardial infarction, renal hypertension and diabetes. This discussion will be divided into topics according to the clinical conditions listed above.
High-fat high-calorie dietA study in Wistar rats assessed the effects of oral gavage administered oleuropein supplementation daily for eight weeks. The rats were divided into three groups: standard diet (2900 kcal/kg), high-cholesterol diet (HCD) (3987.5 kcal/kg), and HCD associated with oleuropein (OL) (50 mg/kg). Oleuropein administration decreased body weight, adipose tissue, triglyceride levels, and liver steatosis.10 Another study that induced adiposity allocated the animals (mice) in three groups for 12 weeks: control with normal diet (NFD; 3865 kcal), high-fat diet (HFD), and HFD supplemented with 0.59% oleuropein (w/w) (or 758 mg/kg) (HFD+OL). Mice fed HFD+OL had significantly lower body weight (31.8%) compared to control, as well as lower serum lipid and triglyceride levels, and no hepatotoxicity was observed.11
Park et al. also showed significant decreases in total cholesterol and triglycerides (TG) with oleuropein supplementation for 10 weeks. The rats were divided into three groups: normal diet, high-fat diet and oleuropein-supplemented diet (0.03% w/w) (OSD). The OSD group, in addition to improved lipid profile, presented a decrease in liver weight.12
Oleuropein supplementation (0.03% for 10 weeks) in C57BL/6N mice fed a high-fat diet (HFD, 40% fat) resulted in reductions in visceral fat and weight gain.13 Similarly, in Olmez et al., male Wistar rats on a high-cholesterol diet were administered ethanolic OLE (50 and 100 mg/kg) by oral gavage for eight weeks. The results indicated that OLE supplementation decreased serum total and low-density lipoprotein (LDL) cholesterol. However, high-density lipoprotein (HDL)-cholesterol and TG levels remained unchanged compared to the control group.14
HypertensionThe effects of OLE supplementation on hypertension have also been studied. Ivanov et al. assessed EFLA® 943 OLE, standardized at 5 (OLE5), 25 (OLE25), and 50 (OLE50) mg/kg OLE in hypertensive rats. OLE5 led to improvement in cardiac and renal hemodynamic parameters, without significant effects on systemic hemodynamic parameters. OLE25 was more effective in reducing cardiovascular risk, improving regional hemodynamics (carotid and renal) and peripheral and regional vascular resistance. OLE50 improved blood pressure and cardiac performance, but tended to retain elevated vascular resistance, thereby reducing the inflow of blood to the rats’ brain and kidneys.15
In spontaneously hypertensive Wistar rats, the effects of chronic consumption of oleuropein-enriched OLE (30 mg/kg) for five weeks on blood pressure, endothelial function, and oxidative and inflammatory status were evaluated by Romero et al. The results included antihypertensive effects such as reductions in systolic blood pressure (SBP) and heart rate (HR) and improved vascular function as a result of reduced pro-oxidative status, restoring eNOS phosphorylation. In addition, OLE reduced levels of reactive oxygen species by reducing NADPH oxidase activity, and reduced expression of vascular toll-like receptor 4 through inhibition of MAPK signaling, which subsequently reduced levels of proinflammatory cytokines.16
A randomized, controlled, double-blind study by Lockyer et al. investigated the effects of OLE on the blood pressure of prehypertensive men (n=60). Dosage was 136 mg oleuropein and 6 mg hydroxytyrosol orally for six weeks with four weeks washout. Daytime (-3.95±11.48 mmHg, p=0.027) and 24-h (-3.33±10.81 mmHg, p=0.045) SBP and daytime (-3.00±8.54 mmHg, p=0.025) and 24-h (-2.42±7.61 mmHg, p=0.039) diastolic blood pressure (DBP) were significantly lower in the OLE group compared to controls. Reductions in plasma total cholesterol (-0.32±0.70 mmol/l, p=0.002), LDL cholesterol (-0.19±SD 0.56 mmol/l, p=0.017) and TG (-0.18±0.48, p=0.008) were also induced by OLE compared to the control group, while a reduction in interleukin-8 (-0.63±1.13 pg/ml, p=0.026) was also detected.17
Heart failureIn a study assessing the effects of OLE on heart failure in rats induced by permanent coronary artery ligation, five groups were analyzed: controls, sham, and 5, 10, or 20 mg/kg/day of oleuropein for five weeks administered by oral gavage. SBP, left ventricular systolic pressure, rate of rise and decrease of left ventricular pressure, and stroke volume, ejection fraction, and cardiac output were significantly lower (p<0.05) than in the control group.18
Similarly, mice had reduced infarction size when treated with 10 or 50mg/g oleuropein 5 min prior to induction of ischemia and beginning of reperfusion.19 Administration of 50 mg/kg oleuropein significantly reduced cardiac ischemia-reperfusion injury, as well as left ventricular end-diastolic pressure during ischemia and reperfusion.20
The effects of administration of oleuropein on ischemia-reperfusion injury (cardiac dysfunction and myocardial infarction) were assessed in male Wistar rats allocated to seven groups, one control and six with interventions of a single dose of 100 mg/kg of oleuropein at one, three, six, 12, 24 and 48 hours prior to excision of the heart. The results obtained showed that single-dose pretreatment with intraperitoneal oleuropein could have a protective effect against ischemia-reperfusion injury for up to three hours, with no significant effect for administration beyond that time.21
Myocardial infarctionAssessing the protective effect of prior administration of oleuropein in rats subjected to myocardial infarction, Janahmadi et al. observed that higher concentrations of oleuropein (20 and 30 mg/kg daily) prevented infarction-induced cardiac dysfunction by increasing cardiac contractility, systolic and diastolic heart function, and increased cardiac output. Oleuropein also raised levels of CK-MB, LDH, troponin I, malondialdehyde (MDA), IL-1β and TNF-α (proinflammatory cytokines), and the antioxidant enzymes superoxide dismutase (SOD) and glutathione reductase (GRx) compared to control.22
Mnafgui et al. studied oleuropein alone administered for seven days in male Wistar rats, aiming to assess its effect in preventing cardiac remodeling after induced myocardial infarction. The findings indicated that treatment with oleuropein was effective in protecting the myocardium, reducing cardiac injury markers, especially troponin T, restoring hemodynamic parameters and attenuating cardiac remodeling.23
Exacerbation of myocardial infarction by acrolein, which is produced by the burning of oils and fats and the incomplete combustion of wood, plastic waste and fossil fuels, and is also found in tobacco smoke, and the attenuating effect of OLE on this outcome, were investigated by Xu et al. Male Sprague Dawley rats received OLE at doses of 200 or 400 mg/kg/day for 28 days, followed by two days of acrolein administration and isoprenaline injection to induce myocardial infarction. The results showed that acrolein aggravated the induced myocardial injury and that pretreatment with OLE significantly (p<0.05) reduced damage to myocardial tissue and infiltration of inflammatory cells, indicating that OLE could prevent the negative effects of acrolein on myocardium and cardiomyocytes.24
Renal hypertension and diabetesThe cardioprotective effects of oleuropein administration in rats with renal hypertension and type 2 diabetes was investigated by Nekooeian et al.25 The study showed that treatment with oleuropein for four weeks is beneficial by increasing the production of antioxidant enzymes such as SOD and lowering MDA, as an index of oxidative stress. In addition to these factors, through ex vivo study of ischemia-reperfusion of the isolated heart, decreased blood pressure, improved contractility and reduced cardiac damage were observed. A similar study with the same doses of oleuropein (20, 40 and 60 mg/kg daily for four weeks) in rats with renal hypertension and type 2 diabetes demonstrated falls in serum total cholesterol, LDL cholesterol, TG and SBP, and increased glucose tolerance and serum HDL and insulin. MDA levels decreased and production of the antioxidant enzyme SOD increased in a dose-dependent fashion.26
The mechanism of oleuropein's antihypertensive effects in animal models (Sprague Dawley rats) with simultaneous type 2 diabetes and renal hypertension was investigated by Nekooeian et al. The animals were divided into five groups, receiving oleuropein at concentrations of 20, 40 or 60 mg/kg/day over four weeks, vehicle only, or non-hypertensive animals (control group). The cardioprotective effects of oleuropein, which might be partly mediated by its antioxidant properties, led to greater release of nitric oxide, as well as antioxidant activity as shown by higher SOD, demonstrating its ability to counteract the physiological mechanisms of disease.27
Study limitationsThree studies were not fully accessed and were therefore not assessed for possible inclusion in the qualitative analysis. Quality assessment was not performed since a validated tool was not available.
ConclusionThis systematic review fills a substantial gap in the literature on the cardioprotective effect of oleuropein in experimental models. The results provide evidence of a positive association between the administration of oleuropein and improvements in hypertension, heart failure, myocardial infarction, renal hypertension and diabetes, and arrhythmias, due to its cardioprotective effect. In view of the promising results of oleuropein's effects on the cardiovascular system in animal models, assessment of its safety and efficacy in humans is called for.
FundingThis research received no external funding.
Conflicts of interestThe authors have no conflict of interest to declare.