Hypertension is the leading preventable risk factor for cardiovascular disease and all-cause mortality worldwide.1 It is estimated that 31% of the world adult population had hypertension in 2010,2 and its prevalence is rising globally due to ageing of the population and increases in exposure to lifestyle risk factors.2 In a multinational study of 63 014 adults from different income countries, 56% of participants were aware of their diagnosis of hypertension, 44% were treated, and only 17% had controlled blood pressure. Awareness and control were less common in upper-middle-income countries, whereas treatment was lowest in low-income countries.3
Randomized clinical trials have demonstrated that blood pressure lowering pharmacological treatment reduces the risk of cardiovascular disease and all-cause mortality.4 A large meta-analysis of 123 clinical trials with 613 815 participants showed that relative risk reductions of cardiovascular disease and all-cause mortality were proportional to the magnitude of achieved blood pressure reductions: every 10 mmHg reduction in systolic blood pressure significantly reduced the risk of major cardiovascular disease events by 20%, coronary heart disease by 17%, stroke by 27%, heart failure by 28%, and all-cause mortality by 13%.4 In addition, it has been estimated that the elimination of hypertension could reduce cardiovascular mortality by 30.4% among males and 38.0% among females.5 Despite this, the rate of hypertensive patients with good blood pressure control is extremely low.3
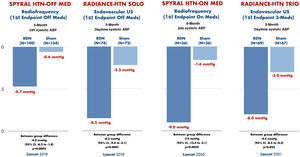
The sympathetic nervous system is a determining component in the development and progression of hypertension.6 Catheter-based endovascular renal sympathetic denervation emerged a decade ago as an option for the treatment, initially, of resistant hypertension.7 Renal denervation (RDN) attenuates signal transmission between the kidney and the central nervous system, resulting in a reduction in renal sympathetic drive, and also reduces postganglionic sympathetic nerve activity, resulting in a reduction in central sympathetic outflow.6 Initial observational studies showed promising results with an improvement in blood pressure control after RDN in patients with resistant hypertension.7,8 However, in 2014, the SYMPLICITY-HTN 3 trial did not demonstrate greater efficacy of RDN vs. a sham-control group to control blood pressure.9 The disagreement between the results of this study and the previous ones, as well as the identification of several confounding factors such as the inexperience of many interventionists, the heterogeneity in the response to RDN in different subpopulations and the interference of drug use in SYMPLICITY-HTN 3,10 highlighted the need to design new studies specifically aimed at solving these questions. Definitive evidence on the efficacy of RDN has come from the SPYRAL HTN and RADIANCE-HTN studies,11–14 all with second-generation devices and optimized RDN technique.6 These trials, with a sham-control group, enrolled patients with and without medical treatment, demonstrating an improvement in blood pressure control in patients treated with RDN, compared with sham control, as shown in Figure 1. Beyond randomized clinical trials, real-life registries have enrolled more than 3500 patients treated with RDN, showing a reduction in both office blood pressure and ambulatory blood pressure monitoring, sustained at three years’ follow up, with a very low rate of complications related to the procedure itself as well as during follow up.15-17
Blood pressure control after renal denervation, compared with sham-control, in four recent randomized clinical trials.
Renal denervation was proven to improve blood pressure control, compared with sham-control, in both patients with and without medical treatment. Adapted from Kandzari D et al., Lancet 2018/Azizi M et al., Lancet 2018/Böhm M et al., Lancet 2020/Azizi M et al., Lancet 2021. ABP: arterial blood pressure; US: ultrasound.
The 2018 European Society of Cardiology and the European Society of Hypertension (ESC/ESH) guidelines for the management of hypertension published in 2018 outlined a role for device-based approaches for hypertension only in the context of clinical trials,17 with the practical effect of discouraging the usage of RDN. Despite the short time that has elapsed since publication of these guidelines, the data provided by the new clinical trials could justify treatment with RDN in selected patients. Two different, but complementary, consensus documents written by experts in hypertension management have been published recently. The ESC/ESH wrote a position paper on RDN with updated recommendations, reviewing new data available about efficacy and safety of RDN, durability of the blood pressure lowering effects of RDN and improvement of cardiovascular outcomes after renal denervation.18 The main recommendations from this position paper are summarized in Table 1. According to these recommendations, the patient's perspective and preference as well as stage of hypertensive disease, including comorbidities, should lead to an individualized treatment strategy in a shared decision-making process, that carefully includes the various treatment options, including RDN.18 The Spanish Society of Hypertension and the Spanish Society of Interventional Cardiology published a joint position statement about RDN centered in strategies to identify potential candidates for RDN according to current clinical evidence, suggesting that patients with uncontrolled hypertension with high cardiovascular risk and hypertension mediated organ damage or established cardiovascular disease could be good candidates.6 This document also makes recommendations about clinical evaluation before RDN, how to perform RDN procedures, and about clinical management of patients after RDN.6 With these new results in patients with moderate hypertension, a new scenario for treatment opens up, so that more and more hypertensive patients will be able to benefit from RDN to control high blood pressure.
Main conclusions and recommendations from the European Society of Hypertension position paper on renal denervation 2021.
| Conclusions/Recommendations | |
|---|---|
| The effects of renal denervation on sympathetic activity | Catheter-based endovascular renal denervation significantly reduces central sympathetic outflow |
| Blood pressure lowering efficacy of renal denervation | In light of second-generation sham-controlled randomized clinical trials, it is now established that renal denervation consistently reduces blood pressure across a variety of hypertensive patients with mild to moderate as well as more severe hypertension, both in the presence and absence of concomitant antihypertensive pharmacotherapy |
| Durability of the blood pressure lowering effect of renal denervation | The antihypertensive effect of renal denervation in humans is durable, although reliable follow-up data are only available for up to three years. Thus, reinnervation does not appear to counteract to durability |
| Does renal denervation improve cardiovascular outcomes? | On the basis of indirect analysis, renal denervation should be considered as an antihypertensive treatment option that reduces blood pressure and contributes to improved cardiovascular prognosis of hypertensive patients |
| Safety of renal denervation | Beyond few femora access complications (hematoma, pseudoaneurysm), no acute adverse safety effects (e.g., acute renal failure, dissections, perforations, bleeding) were observed in the sham-controlled randomized controlled trials. Thus, renal denervation is considered to be a well-tolerated endovascular intervention |
| Renal denervation and open questions | Extensive efforts are ongoing to identify clinical predictors of blood pressure response and thereby to select hypertensive patients that benefit most from renal denervation |
| Pathway to clinical practice for renal denervation | A structured pathway for clinical use of renal denervation is recommended. As healthcare providers, physicians’ perspective and patients’ preference might be discrepant. Implementation of standardized shared decision-making processes to select the best treatment option for blood pressure control, including renal denervation, are suggested. |
Footnote: Adapted from Schmieder RE et al., J Hypertension 2021; 39:1733-1741.
In this issue of Journal, Costa et al.19 report the results from a single-center and their RDN treatment in patients with resistant hypertension. They focus on the role of vitamin D serum concentration as a predictor of blood pressure response. The authors observed a reduction in 24 h mean systolic and diastolic blood pressure in 83% of patients, with a significantly higher baseline vitamin D level in the patients who responded within six months after RDN compared with the patients who did not respond. Identification of reliable peri-procedural and clinical predictors of blood pressure response to RDN remain major unresolved challenges.18 There is a large variability in blood pressure response, as demonstrated by the RADIANCE-HTN SOLO trial. In this trial, individual blood pressure response in daytime ambulatory systolic blood pressure at two months after RDN ranged from an increase in blood pressure of more than 10 mmHg to a decrease of more than 20 mmHg in the RDN group. Similarly, a wide variation in blood pressure responses following RDN has been observed in the other trials.11–14 These preliminary findings provide an interesting line of research to evaluate the real impact of low vitamin D levels in predicting response to RDN.
The large amount of new information available on the efficacy and safety of RDN (there is probably no other context in medicine in which four different randomized clinical trials in which sham control has been performed), with clear positive results in both aspects, surely points to the return of RDN. This leads us to conclude that renal denervation is back and stronger.
Conflicts of interestO. Rodriguez-Leor has received personal fees from Medtronic, outside the scope of the submitted work.