Radiofrequency catheter ablation (RCA) for ventricular tachycardia (VT) in patients with ischemic heart disease (IHD) is associated with a reduced risk of VT storm and implantable cardioverter defibrillator (ICD) shocks. We aim to report the outcome after a single RCA procedure for VT in patients with IHD using a high-density substrate-based approach.
MethodsWe conducted a prospective, observational, single-center and single-arm study involving patients with IHD, referred for RCA procedure for VT using high-density mapping catheters. Substrate mapping was performed in all patients. Procedural endpoints were VT non-inducibility and local abnormal ventricular activities (LAVAs) elimination. The primary end point was survival free from appropriate ICD shocks and secondary end points included VT storm and all-cause mortality.
ResultsSixty-four consecutive patients were included (68±9 years, 95% male, mean ejection fraction 33±11%, 39% VT storms, and 69% appropriate ICD shocks). LAVAs were identified in all patients and VT inducibility was found in 83%. LAVA elimination and non-inducibility were achieved in 93.8% and 60%, respectively. After a mean follow-up of 25±18 months, 90% and 85% of patients are free from appropriate ICD shocks at one and two years, respectively. The proportion of patients experiencing VT storm decreased from 39% to 1.6%. Overall survival was 89% and 84% at one and two years, respectively.
ConclusionsRCA of VT in IHD using a high-density mapping substrate-based approach resulted in a steady freedom of ICD shocks and VT storm.
A ablação por cateter de radiofrequência (ACR) de taquicardia ventricular (TV) em doentes com cardiopatia isquémica (CI) associa-se a redução do risco de tempestade arrítmica (TA) e choques de cardioversor-desfibrihador implantável (CDI). Pretendemos descrever o resultado após procedimento único de ACR de TV na CI utilizando mapeamento de substrato de alta densidade.
MétodosEstudo prospetivo, observacional, unicêntrico e de braço único, envolvendo doentes com CI, referenciados para ACR de TV utilizando cateteres de mapeamento de alta densidade. Os objetivos do procedimento foram a não indutibilidade de TV e a eliminação de atividades ventriculares anómalas locais (LAVAs). O resultado primário foi a análise da sobrevivência livre de choques de CDI apropriados e os resultados secundários incluíram a TA e mortalidade global.
ResultadosForam incluídos 64 doentes consecutivos. Identificaram-se LAVAs em todos os doentes e verificou-se indutibilidade de TV em 83%. A eliminação de LAVAs e a não indutibilidade foram alcançadas em 93,8% e 60%, respetivamente. Após um seguimento médio de 25 ± 18 meses, a sobrevida livre de choques apropriados de CDI a um e dois anos foi de 90% e 85%, respetivamente. A incidência de doentes com TA diminuiu de 39% para 1,6%. A sobrevida global a um e dois anos foi de 89% e 84%, respetivamente.
ConclusõesA ACR de TV na CI, utilizando um procedimento baseado em mapeamento de substrato de alta densidade, associou-se a uma redução consistente dos choques de CDI e TA.
Sustained monomorphic ventricular tachycardia (VT) is associated with an increased risk of mortality and morbidity in patients with ischemic heart disease (IHD).1 While implantable cardiac defibrillators (ICD) have been shown to reduce mortality in patients with IHD and are effective in terminating VT, they are unable to prevent recurrent VT.2,3 Also, recurrent ICD shocks have been associated with an increase in all-cause mortality, hospitalizations for heart failure (HF) and are painful, resulting in impaired quality of life.4–6 Therefore, strategies to prevent ICD shocks are needed.
Adjunctive antiarrhythmic drugs reduce the risk of VT recurrence and ICD shocks; however, they are associated with significant toxicity during long-term treatment, frequently leading to their discontinuation.7,8 Radiofrequency catheter ablation (RCA) is an effective alternative to prevent recurrent VT and ICD shocks in patients refractory or non-tolerant to antiarrhythmic drugs.9 A recent systematic review and meta-analysis of randomized controlled trials (RCTs) reported that RCA for VT in patients with IHD is associated with a reduced risk of VT storm, ICD therapies and, in particular, ICD shocks.10
Ventricular tachycardia frequently results from an underlying reentrant circuit around areas of myocardial patchy scar or at scar borders. Reentry is facilitated by anatomical barriers (fibrosis), functional barriers such as reduced density of gap junctions, reduced gap junction function, and also by anisotropy. Identification of the critical VT protected isthmus is the mainstay of the RCA procedure. Activation and entrainment mapping are able to identify the critical isthmus; however, their use is often not possible due to VT hemodynamic instability. Furthermore, recent work in an animal model suggests that entrainment mapping may overestimate the true size of the isthmus.11 As such, many VTs are unmappable using these techniques and VT ablation often relies on a substrate-based approach, which has been demonstrated to have better results than the previously mentioned mapping techniques.12
The substrate-based approach focuses on the identification and elimination of local abnormal ventricular activities (LAVAs), which can be found during sinus rhythm or pacing at the scar channels that potentially act as critical protected isthmuses during VT. In recent years, new multielectrode mapping catheters with smaller electrodes and interelectrode distance, in association with automatic annotation software, have enabled high-density substrate mapping.13–15 These catheters have improved mapping resolution within areas of low voltage and guided ablation better.16–18 However, there are no RCTs using this novel technology and also there are no long-term results on substrate modification strategies using solely this technology.
ObjectiveThe aim of our study was to report the outcomes after a single RCA procedure for VT in patients with IHD using a high-density substrate-based approach.
MethodsStudy design and populationWe conducted a prospective, observational, single-center and single-arm study from June 2015 to June 2020. We included all consecutive patients with IHD, with or with planned ICD, referred to our center for a first RCA procedure for VT using high-density mapping technology. Inclusion criteria were patients with IHD (history of myocardial infarction (MI) or ischemic scar documented on cardiovascular imaging) with sustained monomorphic VT, documented by surface electrocardiography or ICD tracing and requiring external cardioversion or ICD shock. Patients were excluded if VT was attributable to a reversible cause or if another type of cardiomyopathy coexisted. This research was approved by the local ethics committee. Prior written informed consent was obtained from all participants.
Electrophysiology procedureThe procedure was performed in a fasting state and under conscious sedation. Intra-arterial blood pressure and digital pulse oximetry were monitored continuously. ICD therapies were inactivated. Except for extreme rhythm instability, all antiarrhythmic drugs except β-blockers and amiodarone were discontinued.
A quadripolar non-steerable catheter was placed in the right ventricular apex, and a decapolar steerable catheter was placed in the coronary sinus. Pacing was performed with a pulse width of 2 milliseconds and current of ≥2 times the capture threshold. Left ventricle (LV) access was gained either through an anterograde transseptal approach or by a retrograde aortic approach according to operator discretion. Preference was given to the anterograde approach and the retrograde approach was mainly used in cases of predicted inferior/posterior substrate location (in particular near the subvalvular mitral apparatus). If clinical or preprocedural imaging features suggested an epicardial circuit, epicardial access was performed using a Tuohy needle, guidewire, and deflectable sheath by the Sosa method.19 After LV access was obtained, a bolus of heparin was administered intravenously and repeated as necessary to achieve an activated clotting time of 250-300 s.
Substrate mappingMapping of the LV was performed using three different high-density three-dimensional electroanatomic mapping (EAM) systems and respective multipolar catheters: 1) CARTO 3 and a 20 electrode PentaRay® catheter (Biosense Webster); 2) Ensite Precision and a 16 electrode HDGrid® catheter (Abbot); 3) Rhythmia and a 64 electrode Intellamap Orion® basket catheter (Boston Scientific). The choice of EAM was based on availability at our center. During transmitral mapping, the catheters were supported by an Agilis steerable sheath (Abbot, St Paul, MN).
Substrate mapping was performed during sinus rhythm or while pacing at 600 milliseconds from the proximal electrode of the coronary sinus catheter or from the right ventricle apex, according to operator discretion. Areas of scar (defined as bipolar voltage <1.5 mV) and dense scar (defined as bipolar voltage <0.5 mV) were automatically annotated through adjustment of the voltage thresholds. In patients mapped with the Intellamap Orion® basket catheter an adjusted voltage range of 0.2-0.8 mV was used.20 LAVAs were manually annotated on each substrate map. LAVAs were defined as sharp high-frequency ventricular potentials, distinct from the far-field ventricular electrogram occurring anytime during or after the ventricular electrogram.21 Ripple mapping®, SparkleMap® and Lumipoint® were employed as adjunctive mapping tools to improve the visualization of the scar channels, when the CARTO 3, Ensite Precision or Rhythmia EAM systems were used, respectively.16,22
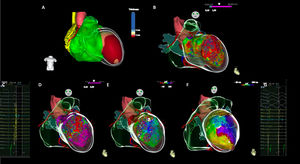
Use of real-time image integration (RTII) was included into the workflow, if available. Cardiac multidetector computed tomography (MDCT) or magnetic resonance (MRI) images were processed using dedicated software (ADAS 3D, Galgo Medical S.L, Barcelona, Spain). Structural substrate was segmented on imaging as areas of wall thinning ≤5 mm on MDCT or delayed gadolinium enhancement on MRI (Figure 1).
High-density multielectrode mapping and real-time image integration in a case of post-anterior myocardial infarction ventricular tachycardia. (A) Real-time image integration using ADAS 3D LV software, demonstrating an anterior and apical left ventricle thickening (≤5 mm). (B) A large anterior and apical scar (<1.5 mV) is shown on endocardial bipolar voltage map, revealing good correlation with RTII. (C) Local abnormal ventricular activities were recorded with a multielectrode catheter (Pentaray). (D) Voltage map during sinus rhythm. (E) Activation map during sinus rhythm depicting late activation on de left ventricle anterior medial segment. (F) Clinical ventricular tachycardia activation map, depicting a slow conducting isthmus on the LV anterior medial segment. (G) Mid-diastolic electrograms recorded with multielectrode catheter (Pentaray) on the left ventricle anterior medial segment, during ventricular tachycardia.
ECG: Electrogram; LV: Left ventricle; LAVAs: Local abnormal ventricular activities; RTII: Real time integration imaging.
After substrate mapping, programmed stimulation from the right ventricle apex at cycle lengths of 600 or 400 ms and up to three extrastimuli down to the ventricular effective refractory period or 200 ms was performed. Isoprenaline was not used for the purpose of inducibility. Induced VTs were classified as clinical or nonclinical based on comparison to the 12-lead electrocardiogram of the clinical VTs. When no 12-lead tracings were available, classification was based on the tachycardia cycle length on ICD tracings. An activation map using EAM and multielectrode catheters was obtained of any induced sustained and hemodynamically stable monomorphic VT. Activation maps during VT were automatically annotated. In regions of low amplitude signal where the far-field signal had been annotated, the annotation was manually adjusted for the local electrogram. Wherever possible, the VT protected isthmus location was confirmed by entrainment techniques. Also, pace mapping was allowed according to operator discretion.
Catheter ablation and procedural endpointsRadiofrequency ablation was delivered with either a sensor enabled open-irrigated catheter with contact force monitoring Thermocool Smarttouch® (Biosense Webster) or a TactiCath Quartz® (Abbot) or without contact force monitoring Intellanav MIFI OI® (Boston Scientific) at 30-50 W endocardial and 25-35 W epicardial.
In the presence of sustained and tolerated monomorphic VT circuits, the VT protected isthmus was the first target for ablation, followed by other regions of LAVAs. In the event of noninducible or hemodynamically not tolerated VT, regions where LAVAs were identified were ablated. The earliest LAVAs were first ablated. After ablation, the LV was remapped with the original mapping catheter, and additional substrate ablation was performed if residual LAVAs were still identified.
Procedural endpoints were VT non-inducibility and LAVAs elimination. Inducibility testing after ablation was avoided if patients were unstable or if they had required ≥2 cardioversions for hemodynamically unstable VT during the procedure.
Post procedural careVenous and arterial sheaths were withdrawn after the procedure. Pericardial sheaths were retracted after obtaining a dry pericardial aspirate and after intra-pericardial corticoid instillation. ICD therapies were reactivated at the end of the procedure. Patients were monitored in hospital for at least 2 days. Antiarrhythmic-drug therapy was continued according to operator discretion.
Follow-upPatients were followed up at one, three, six, and 12 months during the first year and every six months thereafter. Discontinuation of antiarrhythmic drugs was up to the discretion of the treating physician. ICDs were interrogated at each visit and through remote monitoring. ICD programming was left to the discretion of the treating physician. The primary end point was survival free from appropriate ICD shocks. Any documented monomorphic or polymorphic VT terminated by an appropriate ICD shock was considered a recurrence. Secondary end points were repeat procedures, VT storm and all-cause mortality. Indications to repeat a procedure were evaluated on a case-by-case basis. VT storm was defined as ≥3 VT episodes in 24 hours. Mortality during follow-up was categorized as either cardiovascular or non-cardiovascular.
Statistical analysisDescriptive statistics were used to present the results: continuous variables were described by the mean ± standard deviation (SD) or median and interquartile range (IQR) according to the data distribution; and categorical variables were expressed as counts or percentages. The PAINESD mortality risk score after VT ablation was determined to estimate the predicted outcome in this population.23 Kaplan Meier curves were generated to describe ICD shock free survival and overall survival over time. Variables selected from the univariate analyses (p<0.10) were entered into multivariable Cox proportional hazards regression models to estimate predictors of ICD shocks recurrence and overall mortality. All analyses were 2-sided and a p-value <0.05 was considered statistically significant. Statistical analysis was performed by using IBM SPSS Statistics 26™.
ResultsStudy populationFrom June 2015 to June 2020, a total of 64 consecutive patients were referred to our center for a first RCA procedure using high-density mapping for VT. Table 1 and Supplementary Table 1 show the baseline characteristics of the patients. The mean age was 68±9 years, 95% were male. An history of prior myocardial infarction was present in 59 (92%) patients, with a mean time since last MI of 10.8±9.0 years. The remaining five patients had no history of MI and diagnosis of IHD was based on data from cardiovascular imaging. Eighty-three percent of patients were in New York Heart Association (NYHA) functional class II or I and mean left ventricular ejection fraction (LVEF) was 33±11%. Eighty nine percent of patients were taking beta blockers and 78% were taking antiarrhythmic drugs. Eighty three percent of patients had an ICD and 12.5% had undergone a previous VT ablation not using high-density mapping. Thirty nine percent of patients presented with VT storm. The baseline PAINESD risk score was 14±6 and 60.9% were at intermediate risk of acute hemodynamic decompensation.
Baseline characteristics of the patients.a
| N=64 | |
|---|---|
| Patient characteristics* | |
| Age - yr. | 68±9 |
| Male sex - no. (%) | 61 (95.3) |
| Coronary heart disease | |
| Left main disease - no. (%) | 7 (10.6) |
| Multivessel disease - no. (%) | 38 (59.4) |
| Previous percutaneous revascularization - no. (%) | 39 (60.9) |
| Previous surgical revascularization - no. (%) | 14 (21.9) |
| Heart failure and comorbidities | |
| NYHA functional class | |
| I/II - no. (%) | 20 (31.3)/33 (51.6) |
| III - no. (%) | 11 (17.2) |
| LV ejection fraction - no. (%) | 33±11 |
| Atrial fibrillation or flutter - no. (%) | 23 (35.9) |
| Diabetes - no. (%) | 19 (29.7) |
| Moderate or severe renal failure** - no. (%) | 34 (53.1) |
| Chronic pulmonary obstructive disease - no. (%) | 10 (15.6) |
| Cardiovascular medication | |
| Antiarrhythmic drug - no. (%) | 50 (78.1) |
| Amiodarone - no. (%) | 45 (70.3) |
| Sotalol - no. (%) | 5 (7.8) |
| Beta blocker | 57 (89.1) |
| Renin-Angiotensin blockerb - no. (%) | 53 (82.8) |
| Neprisylin inhibitor - no. (%) | 10 (15.6) |
| Mineralocorticoid receptor blocker - no. (%) | 37 (57.8) |
| Antiplatelet therapy - no. (%) | 46 (71.9) |
| Anticoagulant therapy - no. (%) | 25 (39.1) |
| ICD device | |
| Indication and type of ICD before the index procedure - no. (%) | 53 (82.8) |
| Primary prevention - no. (%) | 19 (29.7) |
| Secondary prevention - no. (%) | 34 (53.1) |
| Single-chamber - no. (%) | 25 (39.1) |
| Single-chamber DX - no. (%) | 3 (4.7) |
| Dual- chamber - no. (%) | 16 (25.0) |
| CRT defibrillator - no. (%) | 9 (14.1) |
| ICD implanted after index procedure - no. (%) | 11 (17.2) |
| Indication for ablation | |
| Sustained VT requiring external cardioversion - no. (%) | 20 (31.3) |
| Appropriate ICD shock - no. (%) | 44 (68.8) |
| VT storm - no. (%) | 25 (39.1) |
| PAINESD score | 14±6 |
| Low-risk - no. (%) | 5 (7.8) |
| Intermediate-risk - no. (%) | 39 (60.9) |
| High-risk - no. (%) | 20 (31.3) |
The estimated glomerular filtration rate (GFR) was calculated with the use of the Cockcroft-Gault formula.
Angiotensin-converting-enzyme inhibitor or angiotensin receptor blocker.
CRT: cardiac resynchronization therapy; DX: single-lead ICD capable of atrial sensing via floating dipole; ICD: implantable cardioverter defibrillator; LV: left ventricle; NYHA: New York Heart Association; VT: ventricular tachycardia.
All patients underwent endocardial mapping. A sole transseptal approach was used in 34 patients (53.1%), a sole retrograde in 12 patients (18.8%) and both were used in 18 patients (18.8%). One patient also underwent epicardial mapping: he had previously undergone VT ablation (not using high-density mapping tools) and features suggestive of epicardial circuit were noted in that procedure.
Carto 3 EAM system and the multielectrode PentaRay® catheter were used more frequently (78.1%). RTII using the ADAS 3D LV software was used in 11 patients (17.2%); MDCT was used in six cases and MRI in 6 cases (combined in 1 patient). Median number of points was 2556 (1495). Scar was identified in all patients and represented 39% of the total LV surface area, while a dense scar represented 18% of the total LV surface area. Regions of LAVAs were found in all patients and occupied 10% of the total LV surface area.
Ventricle tachycardia was inducible in 53 patients (83%). Clinical VT was observed in 23 patients (35.9%) and nonclinical VT in 44 patients (68.8%). Activation, entrainment and pace mapping were performed in 24 (37.5%), 3 (4.7%) and 14 (21.9%) patients, respectively.
An open-irrigated catheter with contact force monitoring was used in 58 patients (90.6%), while an open-irrigated catheter without contact force monitoring was used in 6 patients (9.4%). Mean procedure time was 305±65 minutes and mean radiofrequency time was 55±26 minutes. Table 2 and supplementary Table 2 show the procedural data.
Radiofrequency catheter ablation procedural data and outcomes.
| N=64 | |
|---|---|
| Procedural characteristics* | |
| 3D electroanatomic mapping system and multielectrode mapping catheter - no. (%) | 64 (100) |
| Carto 3 and Pentarray- no. (%) | 50 (78.1) |
| Ensite Precision and HD grid - no. (%) | 8 (12.5) |
| Rhythmia and Intellamap Orion - no. (%) | 6 (9.4) |
| Substrate mapping** | |
| Sinus rhythm or atrial pacing/right ventricle pacing - no. (%) | 31 (48.4)/33 (51.6) |
| Number of points - median (IQR) | 2556 (1495) |
| Scar location | |
| Left anterior descending coronary artery territory - no. (%) | 24 (37.5) |
| Circumflex artery or right coronary artery territory - no. (%) | 39 (61) |
| Combined - no. (%) | 1 (1.6) |
| Total LV surface area - cm2 | 230±78 |
| Scar surface area# - cm2 (% Total LV surface area) | 88±60 (39) |
| Dense scar surface area# - cm2 (% total LV surface area) | 40±35 (18) |
| LAVA region surface area - cm2 (% total LV surface area) | 24±17 (10) |
| VT induction and mapping | |
| VT inducible | 53 (82.8) |
| Clinical VT inducible | 23 (35.9) |
| Non-clinical VT inducible | 44 (68.8) |
| Activation/Entrainment mapping - no. (%) | 24 (37.5)/3 (4.7) |
| Pace mapping - no. (%) | 14 (21.9) |
| Procedural data | |
| Total radiofrequency energy delivery time - min | 55±26 |
| Total x-ray exposure time - min | 24±10 |
| Total procedure time - min | 305±65 |
| Procedural endpoints | |
| Assessment of VT inducibility | 47 |
| Clinical VT still inducible - no. (%) | 1 (2.6) |
| Non-clinical VT still inducible - no. (%) | 18 (28.1) |
| Non-inducibility of any VT - no. (%) | 28 (43.8) |
| Elimination of all late potentials - no. (%) | 60 (93.8) |
| Long-term clinical outcome | |
| Appropriate ICD shock - no. (%) | 11 (12.2) |
| Repeat procedures - no. (%) | 4 (6.3) |
| VT storm - no. (%) | 1 (1.6) |
| All-cause mortality - no. (%) | 15 (23.4) |
| Cardiovascular | 9 (60) |
| Noncardiovascular | 5 (33) |
| Unknown | 1 (7) |
Areas of scar (defined as bipolar voltage <1.5 mV) and dense scar (defined as bipolar voltage <0,5 mV). In patients mapped with the Intellamap Orion® basket catheter an adjusted voltage range of 0.2-0.8 mV was used 20.
3D: three dimensional; ICD: implantable cardioverter-defibrillator; IQR: interquartile range; LAVAs: local abnormal ventricular activities; LV: left ventricle; MDCT: multidetector computed tomography; MRI: magnetic resonance; VT: ventricular tachycardia.
Inducibility after ablation was tested in 47 patients: non-inducibility was achieved in 28 patients (60%); 18 patients (38%) had nonclinical VT remaining inducible and one patient remained inducible for clinical VT.
Complete LAVA elimination was achieved in 60 patients (93.8%). In four patients, LAVAs elimination was incomplete. In one case, this was related to anatomy (septal localization); in one case the procedure was shortened due to hemodynamic instability; and in the remaining cases, some LAVAs persisted despite extensive ablation. Table 2 shows the procedural endpoints.
Procedural adverse eventsComplications were observed in eight patients (12.5%). Three patients experienced vascular access complications, one requiring surgical intervention. In two patients, ablation resulted in third-degree atrioventricular block; and two patients experienced cardiac tamponade, a few hours after the procedure, requiring pericardiocentesis. Two patients (3.1%) with advanced HF died within 48 hours after the procedure, as a result of low-flow state.
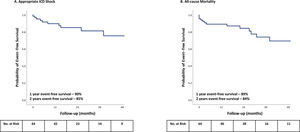
Clinical outcomesPatients were followed for a mean ± SD of 25±18 months after the RCA procedure. Table 2 shows the clinical outcomes. The primary outcome (appropriate ICD shock) occurred in 11 of 64 patients (17.2%). The appropriate ICD shock free survival declined from 90% at one year to 85% at two years (Figure 2). The first appropriate ICD shock occurred during the first year post RCA procedure in 50% of patients (median, eight months; IQR 24), but in 27% it only occurred after three years and up to 4.2 years. Repeat procedures were performed in four of the 11 patients with VT recurrence (36%), including two patients undergoing a combined endo-epicardial procedure.
During follow-up, VT storm occurred in only one patient. The proportion of patients experiencing VT storm decreased from 39% before to 1.6% after a single procedure. The overall survival was 89% and 84% at one and two years, respectively (Figure 2). Throughout the study, 15 patients died (23.4%): 9 died from cardiovascular causes (60%) and 5 died from non-cardiovascular causes (33%). One patient died of unknown cause.
In a univariate analysis, there were no predictors of ICD shock recurrence - Supplementary Table 3. On the other hand, in univariate analysis, age and renal failure were predictors of overall mortality, but they were not significant in multivariate analysis - Supplementary Table 4.
DiscussionMain findingsOur study is the first to report the results after a single RCA procedure for VT in patients with IHD using solely a high-density substrate-based approach with multielectrode catheters.
The main findings were firstly that LAVAs were identified in all patients referred for IHD-related VT ablation, when appropriate and thorough high-density mapping using multielectrode catheters was applied. The end point of LAVAs elimination was feasible (it was achieved in 93.8% of patients) and was effective for arrythmia control. Procedural complications were observed in eight patients (12.5%). Secondly, after a single RCA procedure, 90% of the patients were free from appropriate ICD shocks at one year. The success rate decreased to 85% at two years. First appropriate ICD shock occurred at a median of eight months following the first procedure but spread up to 4.2 years. Also, in 27% of patients, it only occurred after three years, which is longer than the mean follow-up period in most previous studies.24–29 Thirdly, although all-cause mortality was high in this population (23.4%), the majority was related to advanced HF.
Comparison with previous randomized controlled trialsElectroanatomic mappingVentricular tachycardia in IHD is scar-related and results from an underlying re-entrant circuit around areas of myocardial patchy scar or at scar borders. Early coronary intervention for acute MI has led to smaller infarct size and as a consequence, smaller re-entrant circuits and shorter VT cycles. Also, patients with structural heart disease have LV systolic dysfunction, increasing hemodynamic instability during VT. As such, activation and entrainment mapping are only applicable to selected cases and a substrate-based approach using 3D EAM system data should be standard.11,30 In our study, all patients underwent a substrate-based approach. This is in contrast to the majority of previous RCTs, where mapping strategies were underreported (Table 3). A recent meta-analysis found that in patients with structural heart disease related mainly to IHD, there was a significantly lower risk of the composite primary outcome of VT recurrence and all-cause mortality among those undergoing substrate-based ablation.12
Summary of previous randomized controlled trials of radiofrequency catheter ablation of ventricular tachycardia in patients with ischemic heart disease.
| Study, year, N | Electroanatomical mapping* | Procedural endpoint* | Procedural related complications* | Mean follow-up (months) | Appropriate ICD shocks* | VT storm* | All-cause mortality* | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Substrate mapping | Point collection | VT inducibility | Elimination of all late potentials | ||||||||
| Clinical VT still inducible | Nonclinical VT still inducible | Non-inducibility of any VT | |||||||||
| BERLIN-VT, 2020, N=15928 | NR | Multielectrode catheter or point by point using ablation catheter** | 0 | 21.7% | 76.8% | 86.8 | 11.6% | 13±9 | 17.8% | NR | 7.9% |
| SMS, 2017, N=11127 | NR | NR | 6% | 94%# | NR | 7.4% | 28±13 | 14.8% | 7.4% | 16.7% | |
| VANISH, 2016, N=22926 | 88.6% | Point by point using ablation catheter** | 30% | 70%† | 7% | 28± 17 | 37.9% | 24.2% | 27.2% | ||
| CALYPSO, 201525 | NR | NR | NR | NR | NR | NR | 7% | 92% completed 3 months;71% completed 6 months | NR | NR | 9.8% |
| VTACH, 2010, N=10724 | NR | NR | NR | NR | NR | NR | 3.8% | 23± 9 | 26.9% | 25% | 1.9% |
| SMASH-VT,2007, N=12823 | 100% | Point-by-point using ablation catheter | NR | NR | NR | NR | 5% | 23±6 | 9.3% | 6.3% | 9.4% |
The substrate-based approach focuses on identification of low voltage areas and elimination of LAVAs in sinus rhythm or pacing. As such, this approach depends on factors affecting size and shape of electrograms, namely catheter electrodes size and interelectrode spacing. Using very small electrodes with small interelectrode distance in areas of very low voltage, previously considered “dense scar” when mapped using standard point-by-point mapping catheters, surviving myocardial bundles originate sharp and high-voltage signals that can be identified.13–15 In our experience, LAVAs were identified in all patients undergoing mapping using multielectrode catheters. In previous RCTs, the type of catheter used for map collection is underreported (Table 3). When reported, the majority of patients underwent a point-by-point mapping strategy solely with the ablation catheter. Multielectrode catheters enable better mapping resolution within areas of low voltage and improve identification of scar channels, thereby increasing ablation success.16,31 A prior study, adopting a functional substrate mapping strategy based on wavefront discontinuities assessed with these catheters, reported an 80% freedom from VT recurrence at 12±10 months in patients with IHD.18
Real-time image integration, including wall thinning on MDCT and delayed gadolinium enhancement on MRI, correlate with low voltage areas on EAM and the presence of LAVAs.32,33 Also, it has been reported that MRI-aided scar dechanneling is associated with a lower need for radio frequency delivery, higher non-inducibility rates after substrate ablation, and a higher VT-recurrence-free survival.34 In our study, RTII was not systematically used, but it was included into the workflow when available (11 patients, 17%).
Procedural endpoints and complicationsClear and specific procedural endpoints must be established. A meta-analysis of VT ablation in IHD found that the absence of inducible VT at the end of the procedure was associated with a substantially lower risk of recurrent VT.35 Elimination of LAVAs is feasible and safe and is associated with improved survival free from recurrent VT.21 Also, scar dechanneling results in high event-free survival despite the limited ablation extent required.36 Thus, in addition to achieving non-inducibility, elimination of LAVAs and/or scar dechanneling should be pursued. In our series, non-inducibility after ablation was achieved in 28 of the 47 tested patients (60%) and LAVAs elimination was achieved in 93.8% of patients. In previously published RCT there is a clear underreporting of procedural endpoints (Table 3).
Complications are a major concern in RCA of VT in IHD patients. In our series, procedure-related complications were observed in 8 patients (12.5%), while in previous RCT the percentage varied between 3.8% and 11.6% - Table 3. In our study, two patients (3.1%) with advanced HF died within 48 hours after the procedure, as a result of low-flow state. The majority of our patients (60.9%) were at intermediate risk of acute hemodynamic decompensation, as predicted by an increased baseline PAINESD risk score. Periprocedural acute hemodynamic decompensation may be related to the prolonged low-output state related do VT induction and mapping, as well to volume overload due to irrigated catheter ablation and general anesthesia and is associated with an increased risk of mortality. Proper identification of patients at higher risk using appropriate scores such has the PAINESD risk score, and prophylactic mechanical heart support may be valuable to reduce the risk of post procedural adverse events.23,37
Follow-upIn our study, after a mean follow-up of 25±18 months, 90% and 85% of patients were free from appropriate ICD shocks at one and two years, respectively. The proportion of patients experiencing VT storm decreased from 39% to 1.6%. Overall survival was 89% and 84% at one and two years, respectively. Although all-cause mortality was high in our population (23.4%), most deaths were related to advanced HF. A recent systematic review and meta-analysis of RCTs reported similar results, with a reduced risk of VT storm, ICD therapies and, in particular, ICD shocks, albeit no significant difference in all-cause or cardiovascular mortality with RCA for VT in patients with IHD.10 After the index RCA procedure, the mean follow-up ranged from 13–28 months, appropriate ICD shocks and overall mortality occurred in 91 of 375 (24.3%) and in 64 of 391 (16.4%) of the patients included in the ablation arm, respectively (Table 3). RCA procedural characteristics varied between the studies, namely LV access, substrate mapping, VT Inducibility and mapping, ablation and procedural outcomes.
Previous studies found that age ≥70, chronic obstructive pulmonary disease, LVEF <30%, the non-use of multielectrode catheters, non-use of RTII and incomplete substrate ablation were independent predictors of recurrent ICD shocks and overall mortality.31,38 We observed higher mortality in older individuals and among patients with renal failure, albeit these variables did not remain significant in multivariate analysis.
Certainly it will be desirable for future studies to have clear and defined procedural endpoints and outcomes to enable valid comparisons.
LimitationsOur study has several limitations. It was an observational, single-center study, with no control arm to compare diverse mapping and ablation strategies. Additional mapping technologies, namely RTII were only implemented based on availability during the study period. Therefore, the outcome of the entire study cohort may have been hampered by procedures performed at the beginning of the study period. ICD programming and discontinuation of antiarrhythmic drugs were at the discretion of the treating physician. As such, our ablation results have to be interpreted as adjunct to medical therapy and adequate ICD programming and reflect real-world clinical practice.
ConclusionIn this single-center study, radiofrequency catheter ablation of VT in IHD using a high-density substrate-based approach resulted in a steady nonrecurrence of appropriate ICD shocks and VT storm. The adoption of recent improved technical and procedural features, such as high-resolution mapping is fundamental to achieve better success rate in VT ablation.
Conflicts of interestThe authors have no conflicts of interest to declare.