Vitamin D deficiency is a common finding and there is a suggested association with hypertension. Resistant hypertension is a clinical problem observed in 5–30% of hypertensive patients. Renal denervation (RDN) has been used for patients with resistant hypertension and has proven to lower blood pressure. Our primary goal was to assess the vitamin D serum concentration as a predictor of blood pressure response to RDN in highly selected patients.
MethodsThis prospective, nonrandomized, single-center study included 24 patients treated with RDN. Based on their one-year response after RDN, patients were classified as responders or non-responders at six months or at 12 months.
ResultsThe median follow-up was 52 months (range, 14-91 months). After RDN, 17 patients (70.8%) had a reduction >5 mmHg in the mean systolic blood pressure, at the first six months of follow-up. At 12 months, 20 patients (83.3%) were responders. Vitamin D levels at baseline (15.1±4.8 vs. 24.2±8.8 ng/ml) and at six months (16.6±7.2 vs. 25±9.2 ng/ml) were lower in early non-responders compared to early responders (p=0.008), without significant variation during follow-up. Even though Vitamin D levels were lower in the total responder's group, no statistically significant differences were found (p=ns).
ConclusionIn patients with resistant hypertension, low vitamin D concentrations were associated with an absence of early response to RDN.
A deficiência de vitamina D é um achado comum e poderá estar associada a hipertensão. A hipertensão resistente é uma condição clínica observada em 5-30% dos doentes hipertensos. A desnervação renal tem sido aplicada em doentes com hipertensão resistente e tem revelado um decréscimo da tensão arterial. O nosso objetivo é avaliar a resposta preditora da vitamina D na desnervação renal.
MétodosEstudo prospetivo, não randomizado e unicêntrico que incluiu 24 pacientes, entre 38 a 77 anos, tratados com desnervação renal (RDN). A vitamina D foi medida antes do procedimento e durante o seguimento dos pacientes. De acordo com a resposta a um ano, os pacientes foram classificados como respondedores ou não respondedores aos 6 ou 12 meses.
ResultadosO seguimento mediano foi de 52 meses (intervalo, 14-91 meses). Após a RDN, 17 pacientes (70,8%) tiveram uma redução >5 mmHg na tensão arterial sistólica média nos primeiros seis meses de seguimento. Aos 12 meses, 20 pacientes (83,3%) foram respondedores. Os níveis basais de vitamina D (15,1±4,8 versus 24,2±8,8 ng/ml) e aos seis meses (16,6±7,2 versus 25±9,2 ng/ml) foram inferiores nos não respondedores precoces (p=0,008), sem variação significativa durante o seguimento. Apesar de os níveis de Vitamina D serem inferiores no grupo respondedor tardio, não foi encontrado diferença estaticamente significativa (p=ns).
ConclusãoEm doentes com hipertensão resistente, baixas concentrações de vitamina D estão associadas a uma ausência de resposta precoce à desnervação renal.
Vitamin D deficiency is a common finding in 30-50% of the general population.1 Although the consequences of vitamin D deficiency usually involve diseases of the musculoskeletal system, increasing evidence shows there is an association with cardiovascular risk factors, including hypertension (HTN).2 HTN is a major risk factor affecting the global burden of cardiovascular disease.3,4 In spite of lifestyle changes, blood pressure (BP) lowering pharmacological treatments and cardiovascular complications in hypertensive patients, the treatment of HTN remains suboptimal worldwide, and is insufficiently controlled in many patients.5 It is now common knowledge that BP reduction has a favorable impact on prognosis through the reduction of major cardiovascular events such as myocardial infarction, stroke, and cardiovascular death; and hence, the total burden of cardiovascular risk.6,7
The true prevalence of so-called resistant HTN remains unknown, but is reported to range from 5%-30%.8,9 According to the current guidelines of the European Society of Cardiology and European Society of Hypertension, resistant HTN is defined as HTN in patients who do not achieve the targeted BP values despite triple antihypertensive therapy, which includes a diuretic administered at the maximum tolerated dosage.10 Catheter-based RDN is one of the most frequently used invasive method for the treatment of resistant HTN. Some initial non-randomized studies have revealed significant reductions in BP. Nevertheless, the controversial double-blind Symplicity HTN-3 trial did not confirm the superiority of RDN compared to a sham procedure and medical therapy.11 This could be explained by several confounding factors, such as variations in the procedural methods as well as changes in drug regimens after randomization.12–14 Therefore, the recently published Spyral HTN-OFF MED, and the ongoing Spyral HTN-ON MED trials could address these issues and show that RDN could be an effective approach to manage resistant HTN.15,16
Despite some epidemiological data describing the relationship between vitamin D deficiency and arterial HTN,17 evidence investigating the effect of vitamin D is conflicting. There is still debate as to whether vitamin D status influences therapeutical BP reduction.18
Here, our group reports the results of a single center study of percutaneous RDN in patients affected by resistant HTN in daily clinical practice. Our primary goal was to assess the vitamin D serum concentration as a predictor of blood pressure response to RDN in highly selected patients. The secondary goals were to assess the safety and long-term effectiveness of RDN for reducing BP, as well as the echocardiographic response to RDN.
MethodsStudy design and patientsThis was a prospective, nonrandomized, single center study that included 24 patients aged 38–77 years with resistant HTN, who were treated with RDN between May 2014 and October 2017.
A comprehensive medical history and a thorough revision of the medication was undertaken. The initial evaluation included 97 hypertensive patients; 73 were excluded from the study because they did not satisfy the inclusion criteria (Figure 1). The inclusion criteria were as follows: >18 years of age, presence of idiopathic resistant HTN confirmed by ambulatory blood pressure monitoring (ABPM) (mean BP >135/85 mm Hg in spite of a stable regimen of maximum tolerated doses of three or more anti-hypertensive drugs, including a diuretic), glomerular filtration rate >45 mL/min/1.73 m2 (modification of diet in renal disease formula), and compatible renal anatomy (atherosclerotic stenosis <50%, prior renal artery revascularization, fibromuscular dysplasia, or accessory renal arteries as assessed by either computed tomography angiography or duplex ultrasonographic scanning of renal arteries).
The exclusion criteria were as follows: HTN due to secondary causes (screening by biochemical and imaging assessments and polysomnography), hemodynamically significant valvular disease, history of stroke or acute coronary syndrome over the past six months, refusal to sign informed consent, life expectancy <1 year, pregnancy, or presence of pseudo-resistant HTN.
All patients were admitted to the hospital two days prior to the procedure to confirm the presence of ‘true’ resistant HTN by assessing adherence to prescribed medications by witnessed intake of agents. Baseline evaluations included routine blood testing, including Vitamin D concentration, electrocardiography, transthoracic echocardiography, and 24 h Holter monitoring.
The study was approved by the Faculty of Medicine of the University of Coimbra and the Coimbra Hospital and University Center Ethics Committees (reference CE-031/2014. All study patients signed an informed consent.
ProcedureAll RDN procedures were performed by a cardiologist with expertise in endovascular procedures. The standard percutaneous technique used a 6/8 F introducer sheath to enter the femoral artery. A standard JR catheter was used for a selective bilateral renal angiogram performed before and after the procedure. The following three treatment catheters were used (ordered according to chronological use): Symplicity Flex™ catheter (Medtronic Inc, Santa Rosa, CA, USA: two patients), EnligHTN™ (St. Jude Medical, MN, USA: eight patients) and Symplicity Spyral™ (Medtronic Inc, Santa Rosa, CA, USA: 14 patients). A minimum of four and a maximum of 24 ablations that were separated both longitudinally and rotationally and performed in each renal artery. During ablation, the catheter system monitored the tip temperature and impedance and altered the radiofrequency energy in response to a predetermined algorithm. An anesthesiologist was present for all patients. Conscious sedation (via propofol, midazolam, and/or remifentanil) was commonly induced to prevent and manage visceral pain. Intra-arterial heparin and pre- and post-procedure nitrates were administered during the procedure. Hemostasis was achieved using a vascular closure device.
Follow-upThe patients were discharged from the hospital the day after the procedure unless there were immediate complications requiring medical attention. All patients underwent follow-up evaluations at one week, one month, and three, six, 12 months, followed by annual visits. The follow-up evaluations involved a clinical examination, BP measurements in both arms, and medication adjustment, if necessary. Information on adverse events and treatment compliance was recorded. Additionally, follow-up ABPM, electrocardiography, biochemical analysis, including Vitamin D levels, and transthoracic echocardiography were performed at six and 12 months. A renal angiogram was performed at six months in every patient to assess safety. The median duration of follow-up was 52 (range, 14-91) months. According to the response to RDN, the patients were classified as responders or non-responders. A responder to RDN was defined as a patient who obtained >5 mm Hg decrease in the mean systolic BP values determined by ABPM.
Sample calculation and statistical analysisWe planned a study of the continuous response variables, the systolic blood pressure (mm Hg) from matched pairs of study participants. Prior data indicated that the differences between the responses of matched pairs is normally distributed with standard deviation. If the true difference between the mean response of a matched pair is 13, we will need to study 22 pairs of study participants to be able to reject the null hypothesis that the difference is zero with a probability (power) of 0.8. The probability of type I error associated with the test of this null hypothesis is 0.05. Categorical variables were characterized by determining the absolute and relative frequencies, and the numerical variables were characterized by determining the means and standard deviation. The chi-squared test was used for between-group comparisons of the categorical variables. The Mann-Whitney U test was used to compare the continuous variables between two groups, and the Kruskal-Wallis test was used to compare the continuous variables between more than two groups. A general linear model for repeated measures was applied to analyze the variance of each laboratory parameter, which was measured several times in each participant from the responder and non-responder groups. SPSS 19.0 was used for statistical analysis, with a 5% significance level for hypothesis-testing.
ResultsPatient characteristicsA total of 24 patients underwent RDN in our department between May 2014 and October 2017. Based on BP values following the procedure, 20 patients (80.3%) were found to be responders to RDN, of whom 17 (70.8%) were responders at six months. Table 1 shows the baseline demographics and clinical characteristics of the responders and non-responders at six and 12 months.
Clinical baseline characteristics of patients under study according to responsiveness to renal denervation.
| Characteristics | Total (N=24) | 6-month follow-up | 1-year follow-up | ||||
|---|---|---|---|---|---|---|---|
| Responders (N=17) | Non-responders (N=7) | p-value | Responders (N=20) | Non-responders (N=4) | p-value | ||
| Age – years | 59±11 | 61±10 | 55±13 | 0.247 | 60±11 | 56±9 | 0.482 |
| Male - n (%) | 15 (63) | 12 (71) | 3 (43) | 0.356 | 12 (60) | 3 (75) | 1 |
| Time since arterial hypertension diagnosis –years | 17±7.9 | 17.1±8.8 | 17±5.9 | 0.898 | 17.2±8.2 | 16.3±7.5 | 0.907 |
| BMI | 29.7±3.9 | 29.5±3.8 | 30.2±4.5 | 0.691 | 30.1±3.8 | 27.9±4.4 | 0.491 |
| Diabetes - n (%) | 10 (42) | 6 (35) | 4 (57) | 0.393 | 8 (40) | 2 (50) | 1 |
| Insulin therapy- n (%) | 4 (40) | 2 (33) | 2 (50) | 1 | 2 (25) | 2 (100) | 0.133 |
| Smoking - n (%) | 5 (21) | 2 (12) | 3 (43) | 0.126 | 2 (10) | 3 (75) | 0.018 |
| Hypercholesterolemia - n (%) | 22 (92) | 15 (88) | 7 (100) | 0.569 | 18 (90) | 4 (100) | 1 |
| CAD – n (%) | 3 (13) | 2 (12) | 1 (14) | 1 | 2 (10) | 1 (25) | 1 |
| Previous myocardial infarction - n (%) | 2 (8) | 2 (12) | 0 (0) | 0.569 | 2 (10) | 0 (0) | 1 |
| Previous stroke or TIA - n (%) | 5 (21) | 4 (24) | 1 (14) | 1 | 5 (25) | 0 (0) | 0.544 |
| Obstructive sleep apnea - n (%) | 15 (63) | 11 (65) | 4 (57) | 1 | 13 (65) | 2 (50) | 1 |
| CPAP - n (%) | 10 (67) | 9 (82) | 1 (25) | 0.077 | 9 (69.2) | 1 (50) | 1 |
| CKD - n (%) | 2 (8) | 1 (6) | 1 (14) | 1 | 1 (5) | 3 (75) | 0.312 |
| AF - n (%) | 24 (100) | 17 (100) | 7 (100) | NA | 20 (100) | 4 (100) | NA |
| Pacemaker - n (%) | 24 (100) | 17 (100) | 7 (100) | 1 | 20 (100) | 4 (100) | 1 |
| Symptomatic - n (%) | 17 (71) | 11(65) | 6 (86) | 0.384 | 14 (70) | 3 (75) | 1 |
| Number of antihypertensive drugs - n (%) | 5.3±1.1 | 5.2±1.3 | 5.4±0.5 | 0.084 | 5.25±1.2 | 5.5±0.5 | 0.032 |
| Spironolactone - n (%) | 14 (59) | 11 (65) | 3 (43) | 12 (60) | 2 (50) | ||
| CA - n (%) | 24 (100) | 17 (100) | 7 (100) | 20 (100) | 4 (100) | ||
| ARB - n (%) | 17 (71) | 11 (65) | 6 (86) | 14 (70) | 3 (75) | ||
| ACE - no (%) | 10 (42) | 8 (47) | 2 (29) | 8 (40) | 2 (50) | ||
| Thiazide - no (%) | 18 (75) | 13 (77) | 5 (71) | 16 (80) | 2 (50) | ||
| Loop diuretic - no (%) | 6 (25) | 4 (24) | 2 (29) | 4 (20) | 2 (50) | ||
| BB - no (%) | 19 (79) | 13 (77) | 6 (86) | 16 (80) | 3 (75) | ||
| A2AR - no (%) | 15 (63) | 10 (59) | 5 (71) | 12 (60) | 3 (75) | ||
A2AR: alpha2-adrenergic agonist; ACE: angiotensin-converting-enzyme inhibitor; AF: Atrial fibrillation; ARB: angiotensin-receptor blocker; BB: beta blocker; BMI: body mass index; CA: calcium antagonist CAD: coronary artery disease; CKD: chronic kidney disease; TIA: transient ischemic attack.
Analysis of the catheter systems used for RDN show that differences between the systems were not significant. The mean number of ablations performed per each RDN was 25.3±9.10 ablations (a minimum of 10 ablations and a maximum of 41 ablations). Table 2 shows the procedure-related parameters. The differences between procedure-related parameters such as the diameters and lengths of renal arteries, number of renal artery branches, and number of ablations in the responders and non-responders at six and 12 months were not significant.
Characteristics of renal denervation procedure, according to blood pressure response.
| Characteristics | Total (N=24) | 6-month follow-up | 1-year follow-up | ||||
|---|---|---|---|---|---|---|---|
| Responders (N=17) | Non-responders (N=7) | p-value | Responders (N=20) | Non-responders (N=4) | p-value | ||
| Radio-frequency renal denervation system | |||||||
| EnligHTN™ - no (%) | 8 (33) | 6 (35) | 2 (29) | 6 (30) | 2 (50) | ||
| Spyral™ - no (%) | 14 (58) | 10 (59) | 4 (57) | 12 (60) | 2 (50) | ||
| Symplicity Flex™ - no (%) | 2 (8) | 1 (6) | 1 (14) | 2 (10) | 0 | ||
| Maximal diameter right renal artery (mm) | 6.3±1.5 | 6.3±1.6 | 6.2±1.4 | 0.975 | 6.2±1.5 | 6.6±1.8 | 0.51 |
| Minimal diameter right renal artery (mm) | 4.9±1.4 | 4.8±1.5 | 5±1.2 | 0.484 | 4.8±1.4 | 5.5±1.4 | 0.188 |
| Length right renal artery (mm) | 49.9±20 | 47.7±18.8 | 55±23.6 | 0.431 | 50.3±19.4 | 47.4±26.2 | 0.796 |
| Right renal artery ablations (n) | 12.3±4.3 | 12.8±4.6 | 11±3.5 | 0.374 | 12.5±4.6 | 11±2.7 | 0.538 |
| Maximal diameter left renal artery (mm) | 6.3±1.5 | 6.3±1.6 | 6.1±1.5 | 0.634 | 6.1±5.1 | 6.2±5.6 | 0.583 |
| Minimal diameter left renal artery (mm) | 5±1.5 | 4.8±1.5 | 5.5±1.3 | 0.27 | 4.8±1.4 | 6.1±1.5 | 0.113 |
| Length left renal artery (mm) | 42.3±17 | 44.1±17 | 39.3±17.8 | 0.542 | 44.5±16.9 | 33.7±16.1 | 0.254 |
| Left renal artery ablations (n) | 13.2±5.6 | 13.8±5.8 | 11.7±5.1 | 0.426 | 13.8±6.0 | 10.3±1.5 | 0.293 |
| Total ablations (n) | 25.3±9.10 | 26.5±9.67 | 22.4±7.35 | 0.326 | 26.2±9.61 | 20.7±4.03 | 0.088 |
| EnligHTN™ (n) | 21.1±5.8 | 20.7±6.7 | 22.5±2.1 | 20.7±6.7 | 22.5±2.1 | ||
| Spyral™ (n) | 28.9±7.6 | 30.7±7.3 | 25±7.8 | 30.1±6.7 | 15.8±1.6 | ||
| SymplicityFlex™ (n) | 11±1.41 | 10 | 12 | 11±1.4 | NA | ||
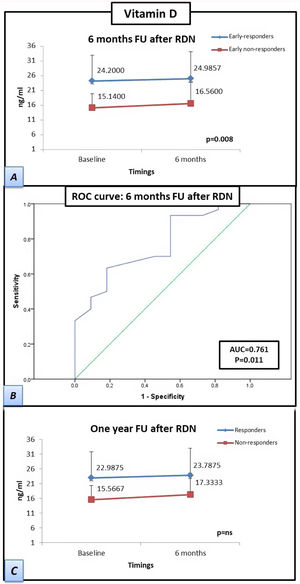
The patients who responded by six months after RDN had a significantly higher baseline vitamin D concentration than the baseline concentration of patients who were non-responders at six months after RDN (15.1±4.8 vs. 24.2±8.8 ng/mL, respectively), which was also observed at the six-month follow-up (16.6±7.2 vs. 25±9.2 ng/mL, respectively; p=0.008). The differences between the vitamin D concentrations of responders vs. non-responders at the 12-month follow-up were not significant. However, the responders at 12 months had higher mean vitamin D levels than those of the non-responders at 12 months both at baseline (15.6±5.1 vs. 23.0±9.0 ng/mL, respectively) and at six months after RDN (17.3±7.6 vs 23.8±9.5 mL, respectively). The receiver operating characteristic curve-derived optimal cut-off for determining response to RDN was ≥19.5 ng/mL (sensitivity 63.3%, specificity 81.8%; p=0.011) (Figure 2).
Patients responding at 6 months to renal denervation have significantly higher levels of vitamin D than patients who do not respond at 6 months. Samples were obtained at twotwopoints (baseline and at six-month follow-up). (A) Estimated marginal mean levels of vitamin D in ‘responders’ at six months (early-responders, blue line) vs. ‘early non-responders’ (red line). (B) Curve of receiver operating characteristics: A vitamin D cut-off level of ≥19.5 ng/ml showed the best overall sensitivity and specificity for determining renal denervation response. (C) Estimated marginal mean levels of vitamin D in ‘responders’ at 1 year after renal denervation (blue line) vs. ‘non-responders’ (red line). Statistical analysis was performed using the Kruskal-Wallis test and a general linear model was applied to analyze the variance of each laboratory parameter. Data are presented as mean ± standard deviation.
FU: follow-up; RDN: renal denervation; AUC: area under the curve; ROC: receiver operating characteristics.
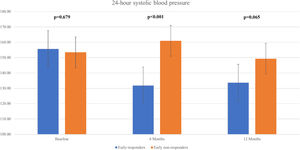
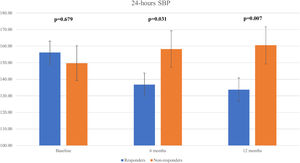
We observed a significant decline in the mean 24 h-systolic BP (SBP) (+10.2 mmHg vs -22.3 mmHg; p=0.007) and 24 h-diastolic BP (DBP) (+5.7 mmHg vs. -13.0 mmHg; p=0.026) in the total responders group, with significantly lower values compared to the non-responders group (Figure 3 and Figure 4). The same findings were detected in both diurnal and nocturnal systolic blood pressure (SBP) and diastolic blood pressure (DBP) values. The differences between the number of antihypertensive agents used in each group at baseline and at six and 12 months of follow-up were not significant.
Regarding long-term follow-up, for a median period of 52 months, seventeen patients (70,8%) remained responders after RDN, one patient died (previous non-responder; cause of death unknown – 4.2%), two responders required new RDN, due to severe BP re-elevation after 18 months’ follow-up, with a positive BP response (8.3%) and four patients remained non-responders (16.7%).
ComplicationsIn terms of safety, new renal artery stenosis was reported in one patient at six-month follow-up. He was a 46-year-old male patient, who had severe refractory hypertension with target-organ lesion (hypertensive nephropathy, stage three chronic kidney disease) and baseline atherosclerotic plaques were identified during baseline renal angiography. The Spyral system was used and a total of 30 ablations were applied (11 on the left renal artery and 19 on the right). No vasospasm was reported post-procedure. At six-month follow-up, the patient presented with flash pulmonary edema, which was treated with stent angioplasty following the identification of new renal artery stenosis (right renal artery, proximal portion, 75% stenosis). The patient had a significant BP reduction after treatment. No other complications were observed.
Impact on echocardiographic variablesThe differences between the variables of the non-responders at baseline and six months after RDN were significant: E/e’ septal (17.7±2.3 vs. 13.8±1.2, respectively; p=0.045), E/e’ lateral (13.3±2.4 vs 10.6±1.9, respectively; p=0.044), and E/e’ mean (14.6±2.6 vs. 11.8±1.8, respectively; p=0.033). No differences between variables were observed between the responders vs. the non-responders. Furthermore, a non-significant decline was detected in the non-responders at 12 months (Table 3).
Echocardiogram characteristics, according to responsiveness to renal denervation, at baseline and at 6-month follow-up.
| Characteristics | 6-month follow-up | p-value | |
|---|---|---|---|
| Responders (N=17) | Non-responders (N=7) | ||
| E/e’ septal at baseline | 12.9±5.4 | 17.7±2.3 | ns |
| E/e’ septal at 6 months | 12.9±7.6 | 13.8±1.2 | ns |
| p-value | ns | 0.045 | |
| E/e’ lateral at baseline | 9.7±2.9 | 13.3±2.4 | ns |
| E/e’ lateral at 6 months | 9.8±3.8 | 10.6±1.9 | ns |
| p-value | ns | 0.044 | |
| E/e’ mean at baseline | 10.6±3.9 | 14.6±2.6 | ns |
| E/e’ mean at 6 months | 11.1±4.8 | 11.8±1.8 | ns |
| p-value | ns | 0.033 | |
In this study, we performed a prospective analysis of the BP-reducing effect of RDN in our center, assessing the predictor value of vitamin D in RDN response. We observed a reduction in the 24 h mean SBP and mean DBP in 83% of the 24 patients. The reduction in BP was comparable with the findings of recent randomized sham-controlled trials. We also observed a significantly higher baseline vitamin D level in the patients who responded within six months after RDN group compared to the patients who did not respond by six months after RDN non-responders. At 12 months after RDN, higher vitamin D values continued to be observed in the patients who responded to RDN, but the difference between the vitamin D levels of the groups was not significant.
Low vitamin D levels have been found to be associated with arterial HTN in cross-sectional studies.19–21 A prospective study found that vitamin D deficiency was associated with an increased risk of HTN, independent of age, body mass index, and other covariates.22 Likewise, observational studies have supported the association between low levels of vitamin D and the presence of resistant HTN.22–24 Various underlying mechanisms to explain this relationship have been discussed. The vitamin D receptor is broadly expressed by cardiovascular tissues that include endothelial cells, cardiomyocytes, and vascular smooth muscle cells.25–27 Vitamin D also suppresses the expression of the renin gene, inhibits the proliferation of vascular smooth muscle cells, and is associated with increased endothelial-dependent vasodilation and reduced cytokine release from lymphocytes. Thus, an association between vitamin D deficiency and HTN may be plausible. Likewise, a recent cross-sectional study found a statistically significant association between vitamin D deficiency and resistant HTN.28 Although there have already been several randomized controlled trials that have examined the effect of vitamin D supplementation on HTN,29–31 there is only one single randomized controlled trial that has assessed the effects of vitamin D supplementation on individuals with resistant HTN. The result was negative,28 however, the study only included 68 patients (34 in each arm), including a some patients with type 2 diabetes. Previous studies have revealed that patients with type 2 diabetes, who were administered vitamin D, achieved reduced BP. Interestingly, in our cohort, the patients who responded after RDN had lower hemoglobin A1C levels than the patients who did not respond to RDN. The results of several studies have suggested an association between the lack of vitamin D and changes in the levels of blood glucose and insulin and the sensitivity of cells to insulin.32 Moreover, an inverse linear relationship between HbA1C levels and vitamin D levels has been reported.32
In our study, the patients who responded by six months after RDN showed a significantly higher baseline vitamin D level compared to the patients who did not respond by six months, which was not significant one year after RDN, although the patients who had responded continued to show a higher mean concentration of vitamin D at one year. The association between low vitamin D concentrations and a decreased response to RDN was previously reported by a Poss et al. in a single retrospective study.33 Nevertheless, they only reported a follow-up of six months, with no further evaluations. To the best of our knowledge, this is the first study to report a lower vitamin D level in patients responding at six months than in patients not responding six months after RDN. To date, it remains unclear whether vitamin D supplementation of patients with resistant HTN can affect the magnitude or timing of responsiveness to RDN. Our findings were observed without the potential effects of calcium supplements, which were neither introduced nor discontinued during this study. Our findings must be considered preliminary only. Additional prospective studies with a larger population of patients are warranted.
We also reported that the patients who did not respond at six months after RDN showed a decrease in the E/e’ septal, E/e’ lateral, and E/e’ mean values, whereas the patients who did respond at six months after RDN did not show a decrease. Most echocardiographic studies find evidence of improved diastolic function after RDN, with divergent results in the diastolic parameters, while others have not been able to find differences between diastolic function over time.34 Additionally, some studies have revealed the beneficial impact of sympathetic modulation on diastolic function without significant association with changes in BP over time.35,36 Interestingly, these studies only considered the patients who were responders, who obtained reduced BP 6 months after RDN. A recent multicenter study found significantly improved ventricular global longitudinal strain, which is a surrogate for diastolic myocardial function, in patients with heart failure. (HF) with preserved ejection fraction who underwent RDN.37 Therefore, additional studies are needed to determine whether RDN could be a treatment option for patients with HF with preserved ejection fraction independent of BP response. Likewise, several studies have confirmed reduction in the left ventricular mass after RDN, independent of changes in BP.35,38 Similar beneficial effects are also suspected for glucose metabolism, obstructive sleep apnea, heart failure, and cardiac arrhythmias.39,40 In our cohort, the LV mass index was not measured. In another Portuguese single center registry of renal denervation, which included 65 patients who underwent RDN, de Sousa Almeida et al. reported a reduction in LV mass assessed in both responders and non-responders at one year follow-up.41 However, in non-responders, no statistical significance was achieved most probably due to small sample size. Regarding diastolic echocardiographic parameters, no significant changes were reported, specifically mitral E/E’ ratio. Nevertheless, the authors did not differentiate between responders and non-responders. Therefore, the data presented in our study about RDN impact on diastolic function should be considered hypothesis generating.
LimitationsOur study has several limitations. It was a single center prospective study with a small sample, there was no control group, and it was not blinded, for RDN (no control group) or for the physicians performing the follow-up echocardiograms. Additionally, different devices were used for RDN, and no specific techniques were used to control patient adherence to medication.
ConclusionThis single center study of patients with resistant hypertension found that RDN was associated with significant reduction in both systolic and diastolic 24 h ABPM blood pressure. There was evidence suggesting a link between vitamin D levels and blood pressure response within six months of RDN. Randomized trials should further clarify whether the normalization of low vitamin D levels with vitamin D supplementation could play a role in the responsiveness of BP to RDN.
Conflicts of interestThe authors have no conflicts of interest to declare.