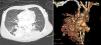
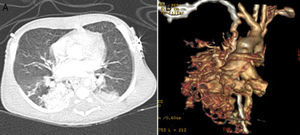
A 23-day-old preterm baby (1900 g) presenting with severe hypoxemia secondary to a large pulmonary arteriovenous malformation (PAVM), diagnosed by thoracic computed tomography (CT), was referred to our department.
He was born at 32 weeks of gestation weighing 1945 g and, after birth, presented with severe hypoxemia (peripheral oxygen saturation [SpO2] 65%). The cardiac assessment was unremarkable. The initial diagnosis was congenital pneumonia with persistent pulmonary hypertension of the newborn and he was treated with antibiotics and ventilated with nitric oxide. In the absence of clinical improvement a thoracic CT scan was performed, which raised suspicion of a PAVM (Figure 1).
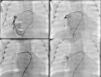
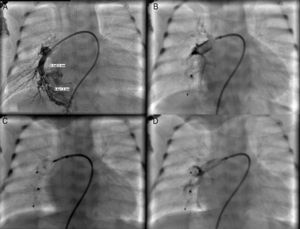
Cardiac catheterization performed under general anesthesia demonstrated a large PAVM in the right lower lobe with two afferent arteries, one 3.1 mm and the other 6 mm in diameter. The first was occluded with a 4 mm Amplatzer® Duct Occluder II (St. Jude Medical, St. Paul, MN) and the other with a 7 mm Amplatzer® Vascular Plug (St. Jude Medical, St. Paul, MN), both implanted through a 4 F delivery sheath (Figure 2). The patient's SpO2 immediately increased to 98% and he was discharged home 20 days after the procedure. One year later, he remains asymptomatic.
(A) Selective angiography of the right pulmonary artery showing a large pulmonary arteriovenous malformation with two afferent vessels; (B) successful closure of the 3.1 mm feeding artery with a 4 mm Amplatzer® Duct Occluder II and of the 6 mm feeding artery with a 7 mm Amplatzer® Vascular Plug (C and D).
This report emphasizes that percutaneous occlusion of PAVM should be the primary therapeutic option at any age. To our knowledge, this is the first such report in the literature in a low birth weight preterm newborn. Amplatzer® devices were the appropriate choice as they fit in a small sheath and are repositionable and suitable for large vessels, ensuring a high occlusion rate.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.