Protein-losing enteropathy is one of the most feared complications of the Fontan circulation. The diagnosis of protein-losing enteropathy in this setting should prompt a thorough investigation for the presence of a treatable hemodynamic impairment. In this report, we describe a complete reversal of protein-losing enteropathy following percutaneous enlargement of a restrictive atrial septal defect in a patient with a fenestrated lateral tunnel Fontan and severe mitral stenosis.
A enteropatia por perda de proteínas é uma das complicações mais temidas da circulação de Fontan. O diagnóstico de enteropatia por perda de proteínas neste contexto deve suscitar uma avaliação exaustiva da eventual presença de uma alteraçao hemodinâmica tratável. Neste artigo, apresentamos uma completa regressão de enteropatia por perda de proteínas após dilatação percutânea de defeito restritivo do septo interauricular num doente com tunel lateral fenestrado de Fontan e estenose mitral grave.
The Fontan procedure is the final step in the staged surgical palliation of patients with a functionally univentricular heart.1 It results in the creation of a circulation in series in which the systemic venous blood is diverted to the pulmonary arteries, bypassing the subpulmonary ventricle.1,2 In order for the Fontan circuit to be achievable, there must be a low resistance to blood flow throughout the circuit.1
Various modifications of the original Fontan procedure have been devised. One of them is the lateral tunnel Fontan, which involves construction of an intra-atrial baffle to direct the inferior vena caval blood to the lungs.1 In addition, a small fenestration can be made in the baffle to provide preload to the systemic ventricle and decompress the systemic venous pathway.2
Protein-losing enteropathy refers to excessive protein loss into the gastrointestinal tract, leading to the development of edema, ascites, pleural effusions, and other complications.2 Clinical presentation and laboratory findings, including low serum albumin levels and increased fecal clearance of alpha-1-antitrypsin, help establish the diagnosis.2
Case reportA 26-year-old female patient presented with a three-week history of increasing dyspnea on exertion, swelling of the legs, and periorbital puffiness that was worse on awakening.
The patient was known to have had double outlet right ventricle, ventricular septal defect, and severe mitral stenosis since birth. She had undergone an atrial septectomy and pulmonary artery banding at one year of age. At the age of 6.5 years, she had had a lateral tunnel Fontan procedure using a size 14 Gore-Tex tube graft with a fenestration, constructed employing Laks’ technique for creating a so-called adjustable atrial septal defect.3 The postoperative course had been uneventful. She had been taking prophylactic acenocoumarol.
On physical examination, there was mild central cyanosis with oxygen saturation of 85% on room air and clubbing. She had mild periorbital and marked pretibial, ankle, and foot edema. The abdomen was slightly distended, and the liver was palpable 3 cm below the right costal margin. Peripheral pulses were normal, and blood pressure was 110/70 mmHg. The heart rate was 98 beats per minute and regular. There was a single second sound and a faint systolic murmur over the left upper sternal border. The lungs were clear on auscultation.
The electrocardiogram showed sinus rhythm, normal axis, right ventricular dominance, and incomplete right bundle branch block. The chest X-ray demonstrated mild cardiomegaly with normal pulmonary vascular markings and clear lung fields. Blood chemistry was notable for decreased total protein and albumin levels (4.9 and 2.4 g/dl, respectively). Twenty-four-hour fecal alpha-1-antitrypsin clearance was significantly elevated (300.8 ml/24 hours), leading to a diagnosis of protein-losing enteropathy.
Transthoracic echocardiography confirmed the above cardiac diagnosis. It also showed a restrictive atrial septal defect (ASD) measuring 4 mm in diameter, with a peak flow velocity of 2.3 m/s across it. The left atrium was slightly dilated, while the left ventricle was moderately hypoplastic. The mitral valve leaflets appeared dysplastic and thickened, with diastolic doming. The mitral valve orifice area was 8.5 mm2. These findings were further confirmed by cardiac magnetic resonance imaging (MRI) (Supplementary Video 1). Both left ventricular end-diastolic volume and left ventricular mass were significantly decreased. The right ventricular cardiac index was calculated to be 2.1 l/min/m2.
Cardiac catheterization was undertaken to widen the interatrial communication. The patient received intravenous heparin at a dose of 50 IU/kg and prophylactic ceftriaxone before the procedure. After obtaining right femoral vein access, a catheter was introduced into the inferior vena cava (IVC). The central venous pressure was 16/14 with a mean of 15 mmHg. There was a mean pressure gradient of 1 mmHg from the IVC to the left pulmonary artery. No pressure gradient was measured from the IVC to the right pulmonary artery.
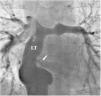
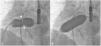
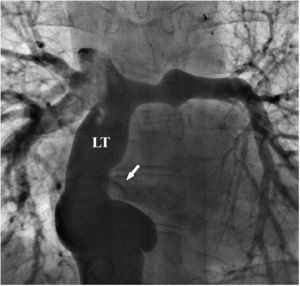
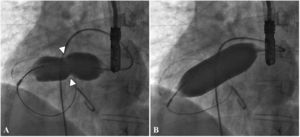
There was a small right-to-left shunt through the 2.3 mm wide fenestration between the lateral tunnel and the left atrium (Figure 1). The catheter was passed into the left atrium through the fenestration. The mean left atrial pressure was measured at 15 mmHg. Intraprocedural transesophageal echocardiography (TEE) revealed a restrictive ASD about 3 mm in diameter with a peak flow velocity of 2.2 m/s (pressure gradient of about 20 mmHg) across it. A guide wire was then advanced from the left atrium across the ASD into the right atrium. The mean right atrial pressure was 11 mmHg. Next, a snare catheter was passed into the aorta via the right femoral artery, and advanced into the right ventricle and the right atrium. The guide wire in the right atrium was grasped by the snare catheter, thus creating an arteriovenous loop. A 20 mm × 45 mm balloon catheter (Balton, Warsaw, Poland) was subsequently inserted over this arteriovenous loop and the balloon was inflated within the ASD. However, the balloon ruptured while fully inflated. The widened ASD now measured about 6 mm in diameter. There was a peak flow velocity of 1.6 m/s (pressure gradient of about 10 mmHg) across the defect as demonstrated by TEE. This result was deemed unsatisfactory and it was decided to repeat balloon dilation with a larger balloon. The catheter was again introduced and the guide wire was passed through the lateral tunnel, fenestration, left atrium, and the ASD into the right atrium. A 25 mm × 40 mm balloon catheter (Osypka AG, Rheinfelden-Herten, Germany) was advanced over the guide wire and positioned across the ASD (Figure 2A). The balloon was inflated until disappearance of the waist on the balloon (Figure 2B). After the septum was torn, the mean pressure gradient between the atria fell from 4 to 2 mmHg. TEE showed a final defect size of 11 mm with laminar flow across it (up to 1.2 m/s).
Follow-up cardiac MRI demonstrated an unrestrictive atrial septum (Supplementary Video 2). In addition, the right ventricular cardiac index increased to 3.2 l/min/m2.
The signs and symptoms of protein-losing enteropathy gradually subsided after the intervention. The patient has been doing well for three years now.
DiscussionProtein-losing enteropathy is a relatively rare complication of the Fontan circulation; however, it is associated with significant morbidity and mortality.2,4 Proposed underlying etiologies include impaired hemodynamics (particularly diminished cardiac output), increased mesenteric vascular resistance, systemic inflammation, and altered glycosaminoglycan content in the basal membrane of enterocytes.5 Protein-losing enteropathy without an established cause remains difficult to treat, despite improvements in medical therapy.4
The diagnosis of protein-losing enteropathy in a Fontan patient necessitates careful investigation for the presence of an underlying hemodynamic disturbance that can be managed.2 Improvements in protein-losing enteropathy have been reported after surgical revision of the circuit, interventional procedures (including percutaneous Fontan fenestrations and balloon dilations with or without stent placement), cardiac pacing, and cardiac transplantation.6–10
In the presence of an atretic or stenotic atrioventricular valve, pulmonary venous outflow has to cross the atrial septum to reach the systemic ventricle.11,12 In such cases, it is crucial to create a non-restrictive interatrial communication at the first stage of palliation. Rarely, if the surgical resection of the atrial septum is inadequate or the rims of the defect become thickened and scarred, obstruction of pulmonary venous outflow at the atrial septal level may occur.13,14
Pulmonary venous outflow obstruction is commonly manifested by persistent pleural and pericardial effusions, ascites, and heart failure.15 Full-blown protein-losing enteropathy may also develop.11,15,16 The diagnosis is usually made by echocardiography. Cardiac MRI may also be helpful.2,12,15 Cardiac catheterization is performed to obtain hemodynamic data and potentially address hemodynamic abnormalities.2,16
Obstruction to flow across the residual atrial septum has traditionally been treated by surgical excision of the atrial septum and unroofing of the coronary sinus.12,13 To our knowledge, however, there is only one description of percutaneous relief of the obstruction across the atrial septum after the Fontan procedure,16 although this might be the preferred treatment modality.
ConclusionProtein-losing enteropathy in patients with Fontan circulation and atretic or stenotic atrioventricular valve may rarely result from obstruction to pulmonary venous outflow at the atrial septal level. In this setting, percutaneous enlargement of the restrictive ASD may be a safe and feasible alternative to surgical resection.
Conflicts of interestThe authors have no conflicts of interest to declare.
The following are the supplementary material to this article: