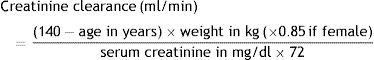Cardiac resynchronization therapy (CRT) has modified the prognosis of chronic heart failure (HF) with left ventricular systolic dysfunction. However, 30% of patients do not have a favorable response. The big question is how to determine predictors of response.
AimsTo identify baseline characteristics that might influence echocardiographic response to CRT.
Methods and ResultsWe performed a prospective single-center hospital-based cohort study of consecutive HF patients selected to CRT (NYHA class II-IV, left ventricular ejection fraction (LVEF) <35% and QRS complex ≥120 ms). Responders were defined as those with a ≥5% absolute increase in LVEF at six months. Clinical, electrocardiographic, laboratory, echocardiographic, autonomic, endothelial and cardiopulmonary function parameters were assessed before CRT device implantation. Logistic regression models were used. Seventy-nine patients were included, 54 male (68.4%), age 68.1 years (standard deviation 10.2), 19 with ischemic etiology (24%).
At six months, 51 patients (64.6%) were considered responders. Although by univariate analysis baseline tricuspid annular plane systolic excursion (TAPSE) and serum creatinine were significantly different in responders, on multivariate analysis only TAPSE was independently associated with response, with higher values predicting a positive response to CRT (OR=1.13; 95% CI: 1.02-1.26; p=0.020). TAPSE ≥15 mm was strongly associated with response, and TAPSE <15 mm with non-response (p=0.005). Responders had no TAPSE values below 10 mm.
ConclusionFrom a range of clinical and technical baseline characteristics, multivariate analysis only identified TAPSE as an independent predictor of CRT response, with TAPSE <15 mm associated with non-response. This study highlights the importance of right ventricular dysfunction in CRT response.
ClinicalTrials.gov identifier: NCT02413151.
A terapêutica de ressincronização cardíaca (CRT) modificou o prognóstico da insuficiência cardíaca (HF) com disfunção ventricular esquerda. Contudo, 30% dos doentes não são respondedores. A grande questão está em identificar preditores de resposta.
ObjetivosIdentificar características basais que podem influenciar a resposta ecocardiográfica à CRT.
Metodologia e resultadosEstudo cohort prospetivo, unicêntrico, hospitalar, de doentes consecutivos com HF selecionados para CRT (classes II-IV NYHA, fração de ejeção ventricular esquerda <35% e QRS≥120 mseg).
Os respondedores foram definidos por aumento absoluto de fração de ejeção ventricular esquerda ≥5% aos 6 meses.
Antes da implantação do ressincronizador, foram avaliados parâmetros clínicos, eletrocardiográficos, laboratoriais, ecocardiográficos, autonómicos, endoteliais e funcionais cardiorrespiratórios. Utilizaram-se modelos de regressão logística.
Incluíram-se 79 doentes, 54 masculinos (68,4%), idade 68,1 (SD=10,2) anos, 19 isquémicos (24%). Aos 6 meses, consideraram-se respondedores 51 doentes (64,6%). Apesar de, por análise univariável, a excursão sistólica do plano do anel tricúspide (TAPSE) e a creatinina sérica serem significativamente diferentes nos respondedores, em análise multivariável, apenas TAPSE foi independentemente associada a resposta, sendo valores superiores preditivos de resposta positiva à CRT (OR=1,13; 95% CI: 1,02-1,26; p=0,020). A TAPSE≥15 mm teve forte associação com resposta, enquanto TAPSE<15 mm a não resposta (p=0,005). Respondedores não tiveram valores de TAPSE inferiores a 10 mm.
ConclusãoDe um conjunto de características basais clínicas e técnicas, a análise multivariável apenas identificou TAPSE como preditor independente de resposta a CRT, associando TAPSE<15 mm a não resposta. Este estudo destaca a importância da disfunção ventricular direita na resposta à CRT.
ClinicalTrials.gov identifier: NCT02413151.
Cardiac resynchronization therapy (CRT) was developed as a treatment for heart failure (HF), and is effective in improving left ventricular (LV) function, with significant impact on the prognosis of symptomatic patients with advanced LV dysfunction and wide QRS.1
Since 2001, the benefits of CRT in terms of reverse remodeling and improvements in symptom severity, quality of life, hospitalization and survival have been clearly demonstrated in randomized controlled clinical trials, as shown in reviews.2 However, despite well-defined selection criteria, the CRT non-response rate, which reaches 30-40% in major trials, still represents a major concern.
Different variables have been studied to determine markers that might predict CRT response.3–12 Echocardiographic measures of ventricular dyssynchrony, for example, cannot identify responders.12
Identifying genuine predictors of CRT non-response remains a challenge.
AimThe study's main purpose was to assess the baseline variables that might significantly influence an echocardiographic response to CRT in HF.
MethodsWe performed a prospective, single-center, cohort study in a central hospital with a multidisciplinary program for HF and CRT, between 2012 and 2014.
Study populationPatients were consecutively selected for CRT according to current guidelines13: New York Heart Association (NYHA) class II-IV, LV ejection fraction (LVEF) <35% and QRS >120 ms.
Inability to perform cardiopulmonary exercise testing (CPET) for any reason, and therapeutic interventions such as exercise training that might confound the results, were considered exclusion criteria.
Study protocolBaseline clinical assessment and testing were performed before CRT device implantation.
An outpatient visit and echocardiogram were scheduled at six months to assess clinical effects and LV reverse remodeling.
A clinical response to CRT was defined as improvement of at least one NYHA functional class and an echocardiographic response as a minimum absolute increase of 5% in LVEF.
Clinical and electrocardiographic parametersAge, gender, body mass index and HF etiology were recorded and cardiac rhythm, heart rate (HR), QRS duration and morphology were determined on the electrocardiogram.
Blood collection and analysisBlood samples were collected in a fasting state for assessment of complete blood count, creatinine, C-reactive protein and brain natriuretic peptide, measured by standard techniques. Creatinine clearance was calculated by the Cockcroft-Gault formula14:
Cardiopulmonary exercise testingCardiopulmonary exercise testing (CPET) was performed under medication using the modified Bruce protocol on a Mortara Multisyn EA 190 treadmill, symptom-limited, with breath-by-breath gas exchange measurements (Innocor).
CPET duration, peak oxygen uptake (peak VO2), percentage of maximum predicted oxygen uptake, exercise ventilatory efficiency, minute ventilation/carbon dioxide production slope (VE/VCO2 slope), anaerobic threshold, peak HR, baseline HR, percentage of maximum predicted HR, double product variation, and difference between peak HR and heart rate recovery index (HRRI), defined as the difference between peak HR and HR at first minute of recovery, were determined.
24-h Holter studyTwenty-four-hour Holter study (Burdick Vision 5L Holter system) included heart rate variation analysis for assessment of the autonomic nervous system. From the time series of NN intervals, a time domain measure, the standard deviation of all N-N intervals (SDNN) was calculated over the 24-h recordings.15
Endothelial function studyEndothelial function was assessed by digital tonometry (Itamar EndoPAT 2000) to study arterial elasticity, with calculation of the reactive hyperemia index (RHI) to determine peripheral arterial tone, according to the EndoPAT protocol.16
Echocardiographic studyTransthoracic echocardiography, including tissue Doppler imaging (TDI) and strain analysis (GE Vivid 9), was performed to assess LV end-diastolic and end-systolic volumes (LVEDV and LVESV, respectively), LV mass (LVM), LVEF (by Simpson's method), global longitudinal strain (GLS), LV inflow E wave and A wave velocities (E and A, respectively), E/A ratio, E/mean e’ wave ratio (E/e’) on mitral annular TDI, tricuspid annular plane systolic excursion (TAPSE), left and right atrial volumes (LAV and RAV, respectively) and pulmonary artery systolic pressure (PASP).
The study protocol was approved by the hospital's ethics committee and complies with the Declaration of Helsinki. Written informed consent was obtained from all patients.
Statistical analysisAn exploratory analysis was carried out for all variables. Categorical data were presented as frequencies and percentages, and continuous variables as means or medians and standard deviation or interquartile range (25th-75th percentile), as appropriate. Chi-square, Fisher's exact and non-parametric Mann-Whitney tests were used, as appropriate. Multivariate analysis was performed using logistic regression models; all variables with a p-value <0.15 in univariate analysis were considered.
The Hosmer-Lemeshow goodness-of-fit test was used, with a high p-value indicating that the model is well calibrated. The Box-Tidwell transformation was used to test the assumption of linearity in the logit of continuous variables and 95% confidence intervals (CI) were calculated as required. The level of significance was considered to be α=0.05.
All data were analyzed using SPSS 22.0 (IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp.).
ResultsOf the 116 consecutive patients referred for CRT, 79 (68.4% male, 24% with ischemic etiology) were included in the study. Four patients were lost to follow-up and 33 were excluded because they were referred for exercise training after CRT or refused to participate in the study.
Patients’ baseline characteristics are summarized in Table 1.
Baseline characteristics of the study population before cardiac resynchronization.
| Variables | n=79 |
|---|---|
| Mean age, years (SD) | 68.1 (10.2) |
| Female gender, n (%) | 25 (31.6) |
| Mean BMI (SD) (min-max) | 27.1 (4.1) (18.0-36.8) |
| Ischemic etiology, n (%) | 19 (24.0) |
| NYHA class, n (%) | |
| II | 23 (29.1) |
| III | 51 (64.5) |
| IV | 5 (6.3) |
| Medication, % | |
| Diuretics | 94 |
| Beta-blockers | 89 |
| ACEIs/ARBs | 90 |
| Anticoagulants | 73 |
| Rhythm, n (%) | |
| Sinus rhythm | 49 (62.0) |
| AF | 27 (34.2) |
| Pacing | 3 (3.8) |
| Median QRS interval, ms (IQR) (min-max) | 140.0 (120.0-160.0) |
| Conduction disturbance morphology, n (%) | |
| LBBB | 77 (97.5%) |
| RBBB | 2 (2.5%) |
| Mean resting HR, bpm (SD) (min-max) | 79.5 (16.5) (45.0-126.0) |
| Mean hemoglobin, g/dl (SD) (min-max) | 13.2 (1.6) (8.7-16.1) |
| Median creatinine, mg/dl (IQR) (min-max) | 1.0 (0.8-1.3) (0.6-2.7) |
| Median CrCl, ml/min (IQR) (min-max) | 68.5 (53.9-91.5) (22.7-146.3) |
| >90, n (%) | 27 (34.2) |
| 60-89, n (%) | 29 (36.7) |
| 30-59, n (%) | 21 (26.6) |
| <30, n (%) | 2 (0.25) |
| Median CRP (mg/dl) (IQR) (min-max) | 2.4 (1.3-6.5) (0.2-75.4) |
| Median BNP, pg/ml (IQR) (min-max) | 286.0 (132.0-782.0) (31.0-2787.0) |
| HRRI, bpm (SD) (min-max) | 15.5 (9.0) (0.0-37.0) |
| Mean CPET duration, msec (SD) (min-max) | 381.4 (248.4) (30.0-947.0) |
| Mean peak VO2, ml/min/kg (SD) (min-max) | 15.1 (5.5) (5.1-31.6) |
| Median VE/VCO2slope (IQR) (min-max) | 37.2 (29.8-46.4) (21.0-71.0) |
| Mean LVEF, % (SD) (min-max) | 26.1 (7.0) (11.0-34.0) |
| Mean LVESV, ml (SD) (min-max) | 152.8 (63.8) (91.0-322.0) |
| Mean LVEDV, ml (SD) (min-max) | 212.2 (68.3) (116.0-420.0) |
| E>A, n (%) | 26/49 (53.0) |
| Mean E/e’ ratio (SD) (min-max) | 18.8 (9.6) (4.0-46.0) |
| Mean GLS, % (SD) (min-max) | -6.4 (3.4) (-18.3-[-1.0]) |
| Mean LVM, g (SD) (min-max) | 351.5 (114.5) (129.0-637.0) |
| Median LAV, ml (IQR) (min-max) | 90.0 (48.3-126.5) (22.0-222.0) |
| Median TAPSE, mm (IQR) (min-max) | 19.0 (16.0-25.0) (5.0-32.0) |
| Median RAV, ml (IQR) (min-max) | 34.0 (20.5-54.5) (9.0-254.0) |
| Mean PASP, mmHg (SD) (min-max) | 40.6 (11.7) (20.0-71.0) |
ACEIs/ARBs: angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; AF: atrial fibrillation; BMI: body mass index, in kg/m2; BNP: B-type natriuretic peptide; CrCl: creatinine clearance (Cockcroft-Gault formula); CPET: cardiopulmonary exercise testing; CRP: C-reactive protein; GLS: global longitudinal strain; HR: heart rate; HRRI: heart rate recovery index; IQR: interquartile range (25th-75th percentile); LAV: left atrial volume; LBBB: left bundle branch block; LVEF: left ventricular ejection fraction; LVEDV: left ventricular end-diastolic volume; LVESV: left ventricular end-systolic volume; LVM: left ventricular mass; NYHA: New York Heart Association; PASP: pulmonary artery systolic pressure; peak VO2: peak oxygen consumption; RAV: right atrial volume; RBBB: right bundle branch block; SD: standard deviation; TAPSE: tricuspid annular plane systolic excursion; VE/VCO2: minute ventilation/carbon dioxide production.
At six months, 54 patients (68.3%) were clinical responders and 51 (64.6%) were echocardiographic responders.
Of the 54 clinical responders, 10 (18.5%) did not respond by echocardiographic criteria and of the 51 echocardiographic responders, six (11.7%) did not improve clinically.
Seventy-seven patients (97.5%) had complete left bundle branch block (LBBB), 50 of them echocardiographic responders (64%). Two patients had complete right bundle branch block (RBBB), one responding to CRT.
Univariate analysis results for baseline variables are summarized in Table 2.
Univariate analysis of variables in terms of echocardiographic response to cardiac resynchronization therapy.
| ≥5% LVEF increase (n=51) | <5% LVEF increase (n=28) | p | |
|---|---|---|---|
| Clinical variables | |||
| Mean age, years (SD) | 68.3 (11.2) | 67.7 (8.4) | 0.571 |
| Female gender, n (%) | 19 (37.3) | 6 (21.4) | 0.148 |
| Ischemic etiology, n (%) | 13 (25.4) | 6 (26.1) | 0.812 |
| Mean BMI (SD) | 27.1 (4.1) | 27.0 (4.1) | 0.975 |
| Median QRS interval, ms (IQR) | 135.0 (120.0-160.0) | 145.0 (127.5-152.0) | 0.865 |
| Sinus rhythm, n (%) | 34 (66.7) | 17 (60.7) | 0.416 |
| Mean resting HR, bpm (SD) | 81.5 (15.0) | 75.8 (18.8) | 0.101 |
| Laboratory variables | |||
| Mean hemoglobin, g/dl (SD) | 13.0 (1.6) | 13.4 (1.6) | 0.447 |
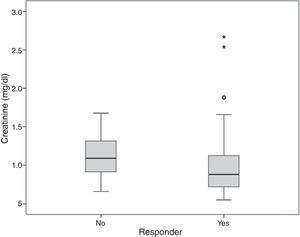
| Median creatinine, mg/dl (IQR) | 0.9 (0.7-1.2) | 1.1 (0.9-1.4) | 0.045 |
| Median CrCl, ml/min (IQR) | 71.6 (55.8-95.4) | 65.3 (52.1-76.9) | 0.191 |
| Median CRP (mg/dl) (IQR) | 2.3 (1.3-5.0) | 3.95 (1.4-9.7) | 0.216 |
| Median BNP, pg/ml (IQR) | 284.0 (97.5-936.5) | 324.5 (219.5-537.0) | 0.673 |
| ANS function | |||
| Median SDNN (P25-P75) | 120.0 (83.5-182.0) | 101.0 (74.0-145.8) | 0.324 |
| Mean HRRI, bpm (SD) | 15.5 (8.8) | 15.5 (9.8) | 0.721 |
| CPET variables | |||
| Mean peak VO2, ml/min/kg (SD) | 14.7 (5.6) | 15.9 (5.1) | 0.387 |
| Mean CPET duration, msec (SD) | 375.4 (253.5) | 393.7 (242.7) | 0.661 |
| Median VE/VCO2 slope (IQR) | 36.6 (30.1-44.1) | 38.4 (28.3-47.8) | 0.992 |
| Endothelial function | |||
| Median RHI (IQR) | 1.5 (1.3-1.9) | 1.5 (1.3-1.8) | 0.508 |
| LV function | |||
| Mean LVEF, % (SD) | 26.1 (7.2) | 25.9 (6.7) | 0.930 |
| Mean LVEDV, ml (SD) | 208.6 (72.7) | 218.6 (60.8) | 0.351 |
| Mean LVESV, ml (SD) | 147.2 (67.9) | 162.8 (55.7) | 0.216 |
| Mean LVM, g (SD) | 348.9 (123.9) | 357.4 (92.1) | 0.607 |
| Mean GLS, % (SD) | -6.7 (3.8) | -5.9 (2.8) | 0.566 |
| E>A, n (%) | 16 (48.5) | 10 (58.8) | 0.488 |
| Mean E/e’ ratio (SD) | 19.6 (10.2) | 17.0 (8.3) | 0.513 |
| Median LAV, ml (IQR) | 67.0 (41.0-123.0) | 101.0 (64.0-140.0) | 0.172 |
| RV function | |||
| Median RAV, ml (IQR) | 34.0 (21.8-58.8) | 30.0 (19.0-52.0) | 0.542 |
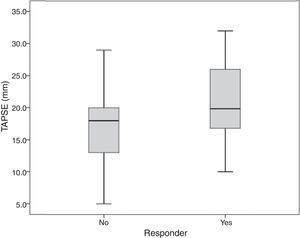
| Median TAPSE, mm (IQR) | 19.9 (16.7-26.0) | 18.0 (12.8-20.5) | 0.031 |
| TAPSE >17 mm, n (%) | 31 (60.8) | 12 (42.9) | 0.119 |
| TAPSE >15 mm, n (%) | 47 (92.1) | 18 (64.2) | 0.005 |
| Mean PASP, mmHg (SD) | 42.4 (12.5) | 37.6 (9.8) | 0.189 |
ANS: autonomic nervous system; BMI: body mass index, in kg/m2; BNP: B-type natriuretic peptide; CrCl: creatinine clearance (Cockcroft-Gault formula); CPET: cardiopulmonary exercise testing; CRP: C-reactive protein; GLS: global longitudinal strain; HR: heart rate; HRRI: heart rate recovery index; IQR: interquartile range (25th-75th percentile); LAV: left atrial volume; LVEF: left ventricular ejection fraction; LVEDV: left ventricular end-diastolic volume; LVESV: left ventricular end-systolic volume; LVM: left ventricular mass; PASP: pulmonary artery systolic pressure; peak VO2: peak oxygen consumption; RAV: right atrial volume; RV: right ventricular; SD: standard deviation; TAPSE: tricuspid annular plane systolic excursion; VE/VCO2: minute ventilation/carbon dioxide production.
Serum creatinine and TAPSE values were associated with echocardiographic response (p=0.031 and p=0.045, respectively) (Figures 1 and 2).
On multivariate analysis only TAPSE was independently associated with echocardiographic response (odds ratio 1.13; 95% CI: 1.02-1.26; p=0.020). TAPSE <15 was associated with non-response (p=0.0052). Eight patients (10%) had TAPSE <14 mm, seven of whom (87.5%) were non-responders. There were no responders (and two non-responders) with TAPSE <10 mm.
DiscussionThere is considerable disagreement in the literature regarding the definition of a CRT responder, as shown by Fornwalt et al.17 in a 26 CRT trials analysis, in which the level of agreement between primary endpoints was poor, with the proportion of patients defined as having a positive CRT response ranging from 32% to 91%.
Clinical response by itself is non-specific, largely subjective and too dependent on different variables. On the other hand, LVEF, although influenced by preload, afterload, HR, and mitral regurgitation, is the most widely used echocardiographic parameter and an important index of LV function, due to its clinical prognostic value.18
In our study, echocardiographic response was defined as ≥5% absolute improvement in LVEF, in order to include the majority of responders. Using a larger LVEF increase would restrict the range of responders, improving response specificity, but decreasing sensitivity. There is still discussion concerning the best LVEF change to identify those who are really responding.17
We observed clinical response in 68.3% of patients and echocardiographic response in 64.6% (non-response in 35.4%).
Demographic and clinical variables did not differ between responders and non-responders. Other investigators also did not find gender or etiology, adjusted to LV volumes, to be independent predictors.8 On the other hand, sub-analyses of randomized clinical trials have suggested that CRT has greater beneficial effects on LV function and/or prognosis in females6,9,10,19 and in nonischemic etiology.7,11
Of our patients, 34% were in atrial fibrillation, the most common arrhythmia in HF and associated with worse prognosis.20 It is unclear if, correcting for age and comorbidity, the prognosis for patients in AF is in fact worse21 or whether it is only a marker of more severe disease.13
We did not find a significant relation between QRS duration and response. Previous randomized controlled trials did not clearly show QRS duration as a predictive factor.22–24 However, in a recent meta-analysis including five randomized trials, QRS duration was a powerful predictor of CRT effectiveness.25 Moreover, a sub-analysis of the MADIT-CRT trial associated longer QRS (>150 ms) with benefits of CRT in LV function, morbidity and mortality.7 The REVERSE study confirmed the importance of QRS duration and LBBB pattern in CRT outcomes.26 With regard to our study, the sample size, the higher percentage of patients with nonischemic etiology and mean QRS >150 ms influenced our results.
In our population, only two patients (2.5%) had RBBB and, although these are generally not expected to benefit from CRT,13 one was a responder.
The only laboratory parameter with statistical significance in univariate analysis was serum creatinine (Figure 1), although creatinine clearance14 showed no association with CRT response. Although lower creatinine levels were seen in patients who responded to CRT (p=0.045), multivariate analysis did not demonstrate an independent association with response.
In contrast to our results, Fung et al.,27 in a population with renal insufficiency, showed that responders had worse baseline renal function. In our study, most patients had normal renal function or mild dysfunction (34.2% and 36.6%, respectively), with only two patients (0.25%) having severe renal dysfunction as defined by the guidelines.28 Different populations in the two studies may have contributed to the difference in results. The relation of serum creatinine and creatinine clearance to response and outcome remains controversial.29
CPET, Holter and peripheral arterial tonometry variables could not identify echocardiographic responders.
Non-responders had greater baseline LV volume, LV mass and left atrial volume and lower LVEF and GLS, although the differences did not reach statistical significance.
In the literature, there have sometimes been conflicting data on the impact of baseline LV dimensions on CRT response, depending on study populations.3,9,10 Park et al. demonstrated that baseline LV volume was a predictor of echocardiographic CRT response.8 Rickard et al.3 showed that the smaller the LV size, the greater the improvement in LVEF and all-cause mortality after CRT. In contrast, the MADIT-CRT trial10 showed that a larger LV was associated with echocardiographic response, and a sub-analysis of PROSPECT9 found no difference in responders.
Regarding the predictive value of baseline LVEF, we found no relation with less severe patients, unlike in the MADIT sub-analysis,30 in which patients with better LVEF had a greater response. However, because different inclusion criteria and definitions of CRT response were used, direct comparisons of results are difficult or impossible.
The right ventricle has always been considered the ‘poor relative’ of the left ventricle, although its importance in exercise tolerance and prognosis has been demonstrated.31 Because CRT was developed to improve LV synchronicity and function, its effects on right ventricular (RV) function have not been fully examined, although some studies have shown benefit.9,32
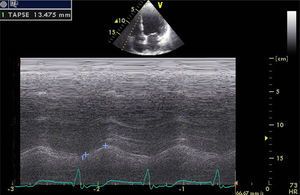
An important issue concerns the potential role of baseline RV dysfunction in LV reverse remodeling after CRT. In our study, TAPSE (Figure 3), a marker of overall RV function33 and an independent predictor of mortality in HF patients,34,35 showed an association with echocardiographic response to CRT by multivariate analysis (Figure 2), with responders having higher baseline TAPSE values. RV dysfunction was present in 7% of responders compared to 36% of non-responders, and TAPSE <15 mm was statistically associated with non-response, although TAPSE <17 mm (the cut-point for RV dysfunction in clinical practice) showed no association. There were no responders with TAPSE <10 mm. This means that only moderate or severe RV dysfunction was related to CRT non-response.
Our results are in agreement with Capelli et al.,36 who demonstrated that CRT induces both RV and LV reverse remodeling, and is more effective in patients with higher TAPSE. In addition, Scuteri et al.32 concluded that low baseline TAPSE was associated with poor response and adverse prognosis, while Sade et al.37 showed that preserved RV function is an independent predictor of long-term event-free survival after CRT.
On the other hand, a sub-analysis of the REVERSE study38 demonstrated that while LV reverse remodeling was greater in patients with TAPSE >14 mm, the difference was not statistically significant. Also, in CARE-HF,39 patients with severely reduced TAPSE (<14 mm) had a significantly lower response rate, though this association was not strong enough to consider TAPSE an independent predictor.
Some of the uncertainty regarding whether TAPSE is an independent predictor of CRT response may result from differences in study populations and particularly in the criteria used to define CRT response.
In conclusion, in this advanced HF population, TAPSE was the only independent predictor of CRT response regarding all the analyzed variables. RV function clearly has a role in the selection of candidates for CRT.
Study limitationsThe study was performed in a single center, and the sample size was moderate, mostly of patients with non-ischemic etiology referred for CRT. Follow-up duration was only six months, even though a small percentage of patients can be late responders. These results need to be reproduced in a larger study, separating patients with ischemic and non-ischemic etiology, with a longer follow-up period.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThis work was supported by research grant PTDC/DES/120249/2010 from the Foundation for Science and Technology (FCT).
Conflicts of interestThe authors declare no conflicts of interest.