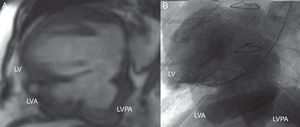
Progressive dyspnea after myocardial infarction can suggests the presence of left ventricular (LV) dysfunction or a left ventricular aneurysm (LVA). Surgical treatment of LVA aims to reduce its volume and to restore the ventricle. Recurrence of LVA after previous repair is extremely rare and the occurrence of concomitant postoperative true and false aneurysms is extraordinary. Surgery is usually challenging because of LV dysfunction and cardiac adherences in reoperations. We describe the simultaneous occurrence in a patient of a recurrent true and false LVA after surgical repair of a postinfarction LVA. Five years postoperatively, the patient remains alive and healthy.
A dispneia progressiva após o enfarte do miocárdio pode sugerir a presença de disfunção ventricular esquerda (VE) ou a formação de aneurisma ventricular esquerdo (AVE). Os procedimentos cirúrgicos concebidos para o seu tratamento visam reduzir o volume e a reparação do VE. A recidiva de AVE após a reparação anterior é extremamente rara e a concomitância de aneurismas verdadeiros e falsos pós-operatórios não é vulgar. A cirurgia é geralmente um desafio por causa da disfunção VE e das aderências cardíacas em reoperações. Apresentamos a concomitância num doente de um AVE verdadeiro e falso recorrente após reparação cirúrgica de um AVE pós-enfarte do miocárdio. Cinco anos após a cirurgia, o doente mantém-se vivo e saudável.
We report the case of a patient previously operated for post-infarction left ventricular aneurysm (LVA) resection. Eighteen years later, he presented a recurrent LVA with, exceptionally, postoperative occurrence of a true and false ventricular aneurysm. He then underwent surgery to reduce and restore the left ventricle. Five years later, the patient remains in good health.
Case reportA 72-year-old male was referred to our institution due to a two-year history of progressive dyspnea. He had undergone inferior LVA resection and left internal mammary artery to left anterior descending artery (LAD) bypass eighteen years earlier. Physical examination revealed a grade II holosystolic apical murmur and the ECG showed previously unnoticed atrial fibrillation. Laboratory findings and chest radiography were unremarkable. Transthoracic echography revealed a dilated left atrium with mild mitral regurgitation. Left ventriculography showed a large bilobulated inferior aneurysm with dyskinetic wall motion (Figure 1A). Left ventricular systolic function was severely depressed, with an ejection fraction (EF) of 0.35 in the non-aneurysmal segments. Coronary angiography showed severe stenosis in the proximal LAD and occlusion of the right coronary artery. The internal mammary graft was patent. Magnetic resonance imaging (MRI) revealed a dilated left ventricle (LV) (163 ml/m2) with severely depressed global contractility (EF 0.20) and a large inferior aneurysm. An additional smaller basal pouch was also identified (Figure 1B).
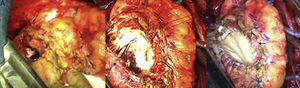
The patient was operated through a median sternotomy. Minimal dissection was performed to allow cannulation of the ascending aorta and right atrium. Due to the firm pericardial adhesions, complete heart dissection was not possible until the heart was completely arrested and the LV decompressed. A true partially thrombosed LVA with linear ventriculotomy reinforced by Teflon strips was identified. With further dissection in the more basal portion of the LV, an infracardiac cavity within the pericardium communicating with the LV aneurysm through a 3 cm×3 cm defect was encountered (Figure 2A). The aneurysmal wall was completely resected (Figure 2B) and the myocardial defect was repaired with an elliptical bovine pericardium patch (Figure 2C). The postoperative course was only complicated by minor psychological disorders and the patient was discharged on the 15th postoperative day. An echocardiographic study performed prior to discharge showed a slightly dilated LV with inferior akinesia and an EF of 0.40. Five years later the patient is alive and in good health.
(A) Intraoperative photographs showing the entrance orifice (arrow) to the false aneurysm in the posterobasal aspect of the left ventricle; (B) intraoperative findings after dissection of the left ventricle, showing the cavity communicating with the previously repaired LVA, and contained by the diaphragm; (C) intraoperative view; arrow shows left ventricular restoration with an elliptical bovine pericardial patch.
Surgically treated postinfarction LVA recurs in less than 5% of patients.1 The most common cause is incomplete scar resection, although extension of myocardial necrosis to surrounding ischemic areas has also been implicated. Left ventricular pseudoaneurysm (LVPA) may also occur after ventricular repair and isolated cases have been reported, mainly after patch repair of left ventricular free wall rupture.2,3 Patch or suture dehiscence or infection and myocardium fragility are the main causes leading to pseudoaneurysm formation. The coincidence of both conditions has very rarely been reported in non-operated patients.4,5 To the best of our knowledge, this association has not been reported after surgical LVA repair.
Unlike true LVA, the natural evolution of LVPA is unpredictable. LVPAs are prone to expansion or rupture, since they lack normal ventricular wall structure. Thus, early intervention is recommended once the diagnosis has been established, especially in large or expanding LVPAs.3
Differential diagnosis between LVA and LVPA is difficult and often requires more than one imaging technique. Diagnosis should be based on the demonstration of discontinuity in the myocardial layer. Furthermore, identification of a neck smaller than the major diameter of the cavity strongly suggests the presence of an LVPA, especially when turbulent flow at the entrance is confirmed by Doppler. Transesophageal echocardiography and especially MR are useful diagnostic tests. Both techniques provide information on the size and location of the defect as well as on valvular and ventricular function. MRI better identifies the different components of the ventricular wall and the presence of thrombi.5
Surgical repair of recurrent LVA and LVPA is technically demanding. The approach is challenging due to the density of pericardial adhesions, the frequent presence of coronary bypass grafts and the usually posterior location of the aneurysm. Whenever a LVPA is suspected the left ventricle should not be dissected until the aorta has been cross-clamped and the heart arrested and decompressed. It is generally accepted that restoring the ventricular wall with a patch is preferable to prevent distortion of the mitral apparatus and excessive tension at the ventricular edges.3,4 Operative mortality is high (20–30%) in this complex population, although the long-term outcome of survivors is satisfactory.4
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.