We present the case of a patient with a high-output fistula between the right superficial femoral artery and femoral vein after left atrial appendage closure successfully treated with a PK-Papyrus covered coronary stent using a 6F guiding catheter. To the best of our knowledge this is the first time a PK-Papyrus coronary stent has been used in this setting.
Apresentamos o caso de um paciente com uma fístula de alto débito entre a artéria femoral superficial direita e a veia femoral, depois de uma oclusão do apêndice atrial esquerdo, tratada com êxito com um stent coronário recoberto PK-PAPYRUS usando um cateter 6F. Até onde sabemos, esta é a primeira vez que o PK-PAPYRUS é o usado neste contexto.
Percutaneous left atrial appendage (LAA) closure devices have been developed as a non-pharmacologic alternative to oral anticoagulant (OAC) therapy for stroke prevention in patients with non-valvular atrial fibrillation (NVAF).1 Vascular access site complications following percutaneous LAA closure are infrequent,2,3 but may be associated with serious consequences.
We present the case of a patient with a high-output fistula between the right superficial femoral artery and femoral vein after LAA closure successfully treated with a PK-Papyrus covered coronary stent using a 6F guiding catheter. To the best of our knowledge this is the first time a PK-Papyrus coronary stent has been used in this setting.
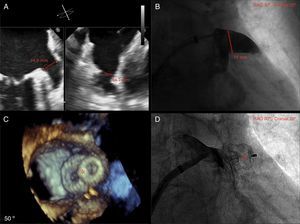
Case reportAn 86-year-old female patient with NVAF, high risk of stroke (CHA2DS2-VASc score of 5) and contraindication to OAC therapy (previous hemorrhagic stroke), underwent percutaneous closure of the LAA with an Amulet device (AGA/St. Jude Medical, Minneapolis, MN). The procedure was performed under general anesthesia, with fluoroscopic and two- and three-dimensional transesophageal echocardiographic guidance. The right femoral vein was cannulated using the Seldinger technique and a transseptal puncture was performed with a Mullins sheath and Brockenbrough needle. A 20 mm Amulet occluder device was successfully deployed (Figure 1).
(A) Left atrial appendage (LAA) size by biplane two-dimensional transthoracic echocardiography (TEE); (B) LAA size by angiography (windsock morphology); (C) three-dimensional TEE showing complete occlusion of the LAA with the Amulet device (red asterisk); (D) angiography showing complete occlusion of the LAA with the Amulet device (red asterisk).
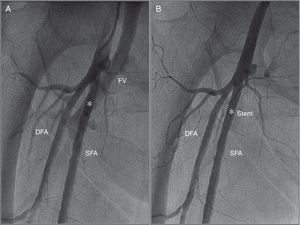
At the end of the procedure after manual compression, prolonged pulsatile bleeding by the femoral venous access was observed. A control angiography obtained from the contralateral (left) femoral artery revealed a high-output fistula between the right superficial femoral artery and femoral vein (Figure 2A). After discussion with the vascular team the decision was made to implant a covered stent. A 26 mm×4.5 mm PK-Papyrus covered coronary stent (Biotronik, Bülach, Switzerland) was inserted via the left femoral artery and deployed at the arteriovenous (AV) fistula using a 6F multipurpose catheter (Cordis Corporation) and a coronary guidewire (Balanced Heavyweight, Abbott Vascular Inc.). At final angiography, complete sealing of the AV fistula was documented (Figure 2B).
(A) Angiography obtained from the contralateral femoral artery (left) revealing a high-output arteriovenous fistula between the right superficial femoral artery and femoral vein (white asterisk); (B) angiography after implantation of a 26 mm×4.5 mm PK-Papyrus covered coronary stent (white asterisk) revealing complete sealing of the AV fistula. DFA: deep femoral artery; FV: femoral vein; SFA: superficial femoral artery.
The patient was discharged after 48 hours without complications. Six months after the procedure, she was asymptomatic and Doppler ultrasonography of the right femoral segment was normal.
DiscussionPercutaneous LAA closure is a demonstrated alternative strategy to OAC therapy for stroke prophylaxis in patients with NVAF.1 Vascular access site complications (perforations, pseudoaneurysms or AV fistulas) following percutaneous LAA closure are infrequent,2,3 but require immediate assessment and management and may be associated with adverse outcomes.
These vascular complications can be treated percutaneously using covered stents with high technical success, thus avoiding high-risk urgent vascular surgery.4–6 To the best of our knowledge this is the first time a PK-Papyrus coronary stent has been used in this setting.
The PK-Papyrus covered coronary stent is a sixth-generation bare-metal stent made of a cobalt-chromium alloy, which allows for thinner struts while still maintaining optimal radial strength and radiopacity. The polyurethane membrane covering the stent is only 90 μm thick. The wide size range (diameter 2.5-5.0 mm, length 15-26 mm) enables a broad range of vessels to be treated confidently and efficiently. Many current covered stents designed for peripheral interventions require large guiding catheters to be deployed. The PK-Papyrus stent is compatible with 5F- and 6F-guiding catheters (recommended sizes are 5F for 2.5-4.0 mm stents and 6F for 4.5-5.0 mm stents), and thus may reduce vascular complications related to the large (>6F) catheters and sheaths frequently used in these interventions. In addition, the techniques and materials used for the implantation of a PK-Papyrus covered stent in a peripheral segment are the same as used in coronary interventions.
ConclusionAV fistulas following percutaneous LAA closure are infrequent, but may associated with adverse outcomes. The PK-Papyrus covered coronary stent can be a good choice for percutaneous treatment of these complications.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.