The field of three-dimensional printing applied to patient-specific simulation is evolving as a tool to enhance intervention results. We report the first case of a fully simulated percutaneous coronary intervention in a three-dimensional patient-specific model to guide treatment.
An 85-year-old female presented with symptomatic in-stent restenosis in the ostial circumflex and was scheduled for percutaneous coronary intervention. Considering the complexity of the anatomy, patient setting and intervention technique, we elected to replicate the coronary anatomy using a three-dimensional model. In this way, we simulated the intervention procedure beforehand in the catheterization laboratory using standard materials. The procedure was guided by optical coherence tomography, with pre-dilatation of the lesion, implantation of a single drug-eluting stent in the ostial circumflex and kissing balloon inflation to the left anterior descending artery and circumflex. Procedural steps were replicated in the real patient's treatment, with remarkable parallelism in angiographic outcome and luminal gain at intracoronary imaging.
In this proof-of-concept report, we show that patient-specific simulation is feasible to guide the treatment strategy of complex coronary artery disease. It enables the surgical team to plan and practice the procedure beforehand, and possibly predict complications and gain confidence.
O campo da impressão tridimensional aplicado à simulação específica de doente está a evoluir como uma ferramenta para aperfeiçoar os resultados das intervenções terapêuticas. Pretendemos descrever o primeiro caso de simulação completa de intervenção coronária percutânea, num modelo tridimensional específico de doente, para guiar o procedimento.
Uma doente de 85 anos com reestenose intra-stent sintomática no óstio da artéria circunflexa foi referenciada para intervenção coronária percutânea. Considerando a complexidade da anatomia, o contexto clínico e a técnica de intervenção, decidimos reproduzir a anatomia coronária com o uso de um modelo tridimensional. Desse modo, simulámos antecipadamente a intervenção no laboratório de hemodinâmica com material habitual. O procedimento foi guiado por tomografia de coerência óptica, com pré-dilatação da lesão, implantação de um stent farmacoativo na circunflexa ostial e insuflação kissing balloon na descendente anterior e circunflexa. As etapas do procedimento foram reproduzidas no tratamento real, com um paralelismo notável nos resultados angiográficos e ganho luminal avaliado por imagem intracoronária.
Com esta prova de conceito, demonstramos que a simulação específica de doente é exequível para guiar a estratégia de tratamento de doença coronária complexa, possibilitando o planeamento antecipado do procedimento, possivelmente prevendo complicac¿o¿es e adquirindo mais confianc¿a.
An 85-year-old female came in to the outpatient clinic for a follow-up visit complaining of a two-month history of typical chest pain on exertion (grade II-III according to the Canadian Cardiovascular Society classification) and progressive dyspnea. Her cardiovascular risk factors included hypertension, dyslipidemia and obesity. Past medical history was significant for coronary artery disease with hospitalization for unstable angina three years before. At that time, ejection fraction was normal and coronary angiography showed mild distal left main disease and severe diffuse stenosis of the right coronary artery (RCA). Four drug-eluting stents (DES) were implanted in the RCA with good acute angiographic result. After hospital discharge she was doing well, without any symptoms. Treatment included acetylsalicylic acid 100 mg, lisinopril 20 mg, nebivolol 5 mg and pitavastatin 2 mg daily. A nitroglycerin transdermal patch was added to her usual therapeutic regimen and she was scheduled for invasive stratification. At coronary angiography, mild diffuse restenosis of the RCA stents was found. An intermediate lesion was detected in the distal left main (LM) coronary artery affecting the circumflex (Cx) ostium. There was also severe calcification of the left anterior descending (LAD) artery with intermediate lesions in the proximal and mid segments. We proceeded to functionally evaluate the patient with a Verrata® pressure guide wire (Philips Volcano, USA), yielding an instantaneous wave-free ratio (iFR®) of 0.79 in the mid-LAD and 0.91 in the proximal Cx. Pullback iFR® analysis from the LAD showed that the only significant step was in the proximal LAD. Therefore, we decided to perform focal percutaneous coronary intervention (PCI) of that lesion. With a protection wire in the Cx, and following pre-dilation of the LAD with a scoring balloon, a DES was implanted in the proximal LAD (2.75×22 mm). The procedure was complicated by distal LM dissection propagating to the Cx, probably induced by deep engagement of the extra backup guiding catheter. The patient was hypotensive, but flow was restored after kissing balloon dilation in the distal LM bifurcation. After that, we used a two-stent mini-crush technique considering Cx as a side branch using a 3.5×18 mm DES and stenting LAD to LM using a 3.5×30 mm DES overlapping the other LAD stent. Kissing balloon inflation was followed by the proximal optimization technique of LM with a 5-mm balloon. The LAD to LM procedure was guided by intravascular ultrasound (IVUS) imaging. There was good stent expansion and apposition on IVUS, as well as good angiographic result. Furthermore, the final iFR on the LAD distal to stents was 0.91. The patient remained stable during hospital stay and was discharged with double antiplatelet therapy.
After four months, the patient presented with recurrent angina on exertion. Therefore, we performed a coronary angiography, which showed good result of the previous PCI of the LM to the LAD and RCA, but there was a critical, focal, in-stent restenosis of the ostial Cx. At that point, we decided to stage the procedure in order to plan the treatment strategy.
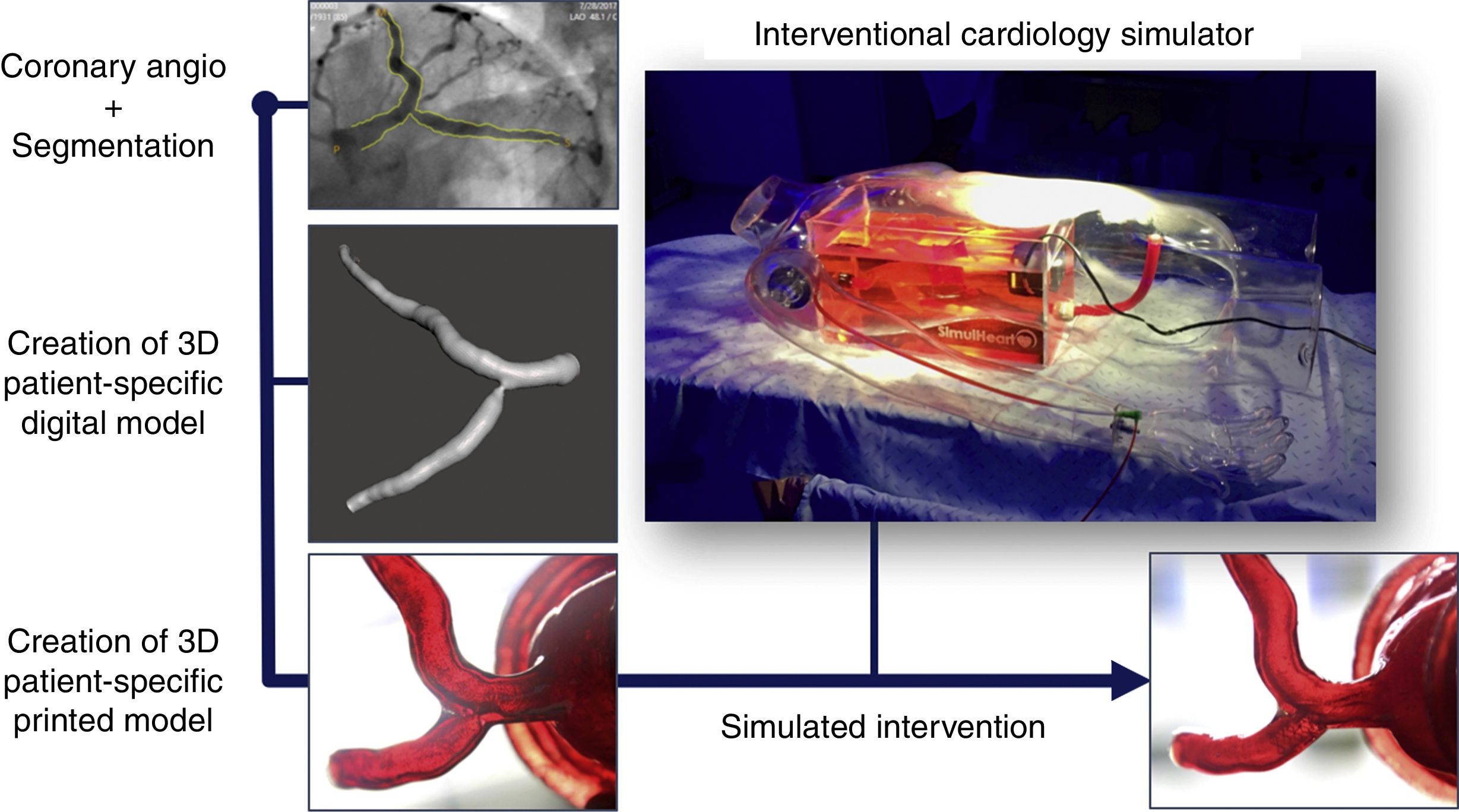
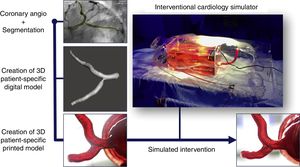
As our group has experience in cardiac three-dimensional (3D) printing and simulation, we decided to test the treatment strategy for this complex PCI beforehand with the use of a 3D patient-specific simulator. Briefly, we used two-dimensional angiography data to render a 3D volume depicting the proximal left coronary artery using CAAS QCA-3D software (Pie Medical Imaging BV, the Netherlands) and digitally added parts to the model in order to connect it to the simulator (Figure 1). The coronary anatomy was then printed in 3D using a stereolithography printer1 in order to obtain a final patient-specific coronary artery model made of custom hybrid flexible material, with a dual-layered design and filled with fluid. Finally, we connected the coronary 3D-model to our custom-made interventional cardiology simulator, the SimulHeart® (Coimbra, Portugal). As a brief summary, the SimulHeart® is a realistic interventional cardiology simulator to be used in standard catheterization laboratories. Its main features include 3D-printed vascular anatomy, as well as radial and femoral access sites that enable the use of actual diagnostic and interventional devices with realistic haptics feedback (Figure 1).
The steps for creating a patient-specific 3D coronary model for simulation are depicted. They include segmentation of the coronary angiography in order to create a 3D patient-specific digital model, and post-processing to obtain a flexible and transparent patient-specific physical model that could be connected to the custom interventional cardiology simulator SimulHeart®. The images on the bottom show the ostial circumflex stenosis model on visual inspection (left) and the post-intervention result after stent placement (right).
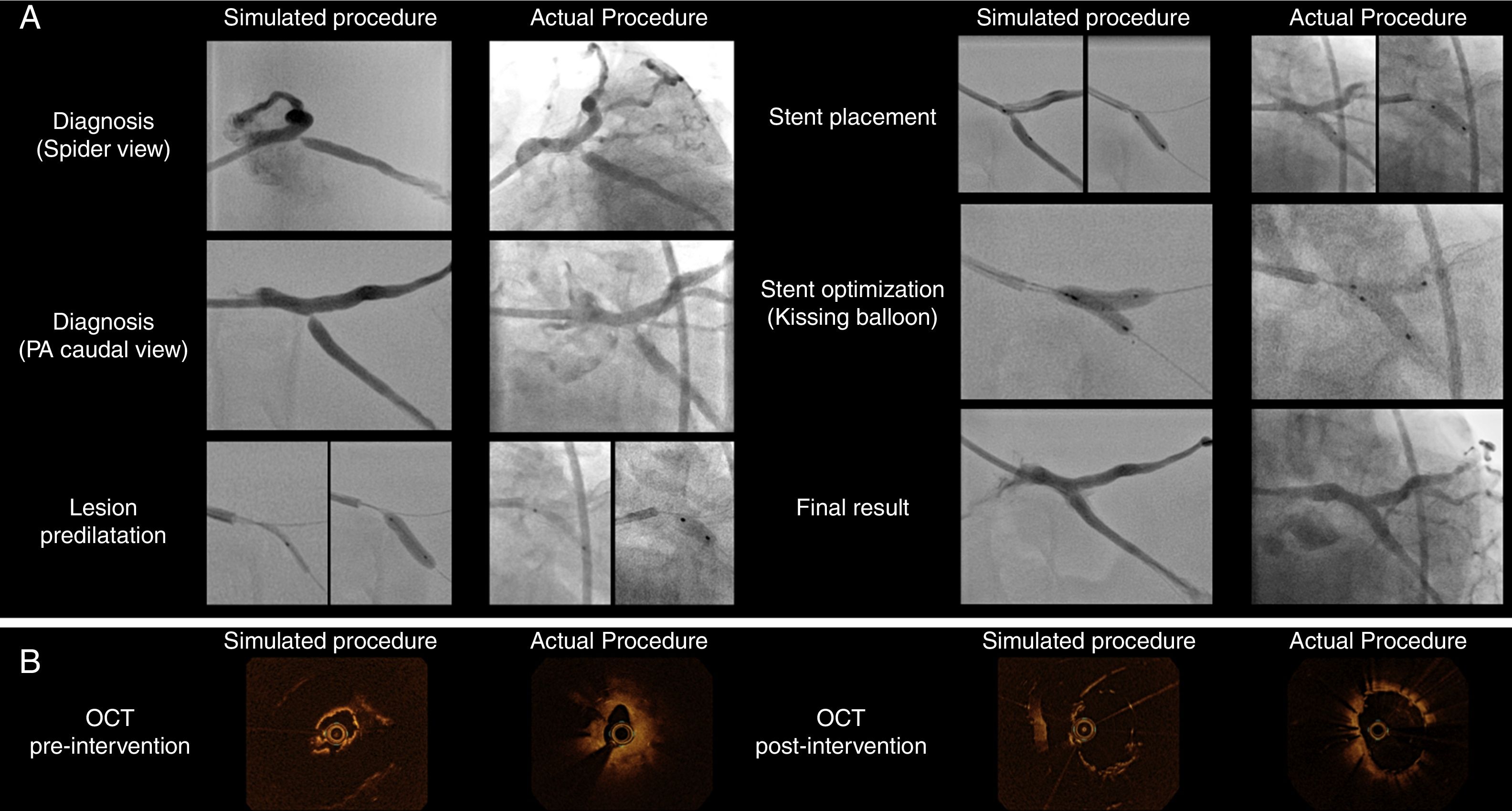
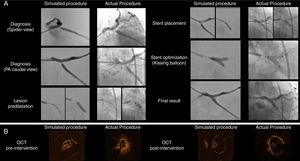
The patient-specific PCI simulation was guided by optical coherence tomography (OCT) imaging, using a Dragonfly® catheter (Abbott, Chicago, Illinois, USA). The right radial access was used to engage the left main with a 6 Fr extra backup guiding catheter. The Cx and LAD were wired and standard sized measurements for landing zones were obtained via OCT. Then, the ostial Cx stenosis was pre-dilated with 2.5- and 3.0-mm balloons, followed by the deployment of a 3.5×9-mm DES in the Cx ostium with slight protrusion to the left main stem. Then kissing balloon inflation was used to better shape the carina. Final OCT images showed good stent expansion with a small number of struts protruding to the LM, which was considered a good result for the predefined primary treatment strategy. The angiographic and OCT images of the simulated procedure are depicted in the left-hand boxes in Figure 2. Also, at the right bottom part of Figure 1, the result of stent implantation can be seen by directly inspecting the transparent, flexible patient-specific 3D print.
Side-by-side display of matched angiographic appearance (Panel A) and OCT imaging (Panel B) of patient-specific simulated procedure and actual procedure for comparative purposes. (Panel A) Angiographic images depicting the sequential procedural steps from diagnosis to final surgical result. (Panel B) OCT still frames of minimal luminal area before intervention and the final result after stent implantation.
The following day, we performed the actual procedure on the patient, keeping in mind the planned steps from the simulation. However, the right radial pulse was absent and, therefore, we used the right femoral arterial access. OCT imaging showed that the mechanism of restenosis was stent under-expansion at the Cx ostium where there was significant plaque burden that may have had limited expansion previously. Therefore, we considered that another stent was needed to reshape the vessel and minimize the recurrence of restenosis. We used a similar selection of interventional material and carried out the same sequence of steps to perform this PCI as tested the day before, achieving a good result both angiographically (Figure 2, Panel A) and on OCT imaging (Figure 2, Panel B). The procedure was uneventful and the patient was discharged the next day. Figure 2 compares the simulation and actual procedures. The parallelism in angiographic outcomes and also the similarities in luminal gain in OCT are noteworthy.
DiscussionSimulation has evolved as a learning tool and has been shown to be effective for teaching both novice and experienced learners. Interventional cardiology learning curves and the volume-outcome relationship suggest that simulation may be used to potentially improve clinical results. However, several hurdles prevented it from being widely adopted in the past. Nowadays, 3D printing is emerging2 and may be a game-changing technology to replicate vascular anatomy and enable simulation experiences.
In particular, the exciting field of patient-specific simulation is in the early development phase, mainly in the surgical training context. A recent systematic review on this matter confirms the feasibility of patient-specific simulators and the authors hypothesize that in the future it may be able to support training of higher-level competencies. By testing a patient-specific case, the interventionist and his team could familiarize themselves with the case, try different approaches, identify potential risks, reduce radiation dose and optimize tool selection.3 A trial is currently underway for the assessment of patient-specific simulators for endovascular aneurysm repair training (NCT02372214). However, there are few reports on patient-specific simulations for vascular percutaneous intervention. As an example, carotid artery stenting can be tested in a virtual reality simulation with a high degree of similarity with the tools and angiographic results of the actual procedure.4 However, these simulators lack the tactile experience that is paramount in percutaneous procedures. On the other hand, 3D simulators offer a physical experience in an accurate patient-specific anatomy. Itagaky et al. treated multiple splenic artery aneurysms in a 3D-printed model, and then used the same combination of guide catheter, base catheter and microcatheter to successfully treat the patient, with minimized radiation dose.5 In a benchmark validation study, two cases of endovascular aortic aneurysm repair were compared with patient-specific stent deployment 3D models. By comparing stent positions in simulations and post-operative scans, the authors determined the simulation-predicted stent locations and shapes with an accuracy of a few millimeters.6 In the field of complex structural heart intervention, 3D printing has been used to select device sizes, although not in a fully simulated procedure setting.7
To the best of our knowledge, this is the first report of simulated coronary PCI in a 3D patient-specific model. Considering patient frailty, complexity of the anatomy and previous bifurcation PCI, we decided to replicate the coronary anatomy using a 3D model to simulate the intervention procedure in the catheterization laboratory using standard materials.
Computed tomography, magnetic resonance imaging or 3D echocardiography data sets have often been used to render the 3D volumes.8 However, 3D angiography based on planar angiographic images may be useful. These methods are familiar to interventional cardiologists and they may provide a more detailed anatomy due to providing higher spatial resolution than non-invasive modalities. However, their reliability may be impaired in highly eccentric plaques (which was not our case) because they use only two projections to derive the 3D geometry of the vessel of interest.
According to the European Society of Cardiology guidelines, it is advisable to treat in-stent restenosis with either DES or drug-coated balloons (class I, level of evidence A).9 In fact, drug-coated balloons were second only to everolimus-eluting stents to treat in-stent restenosis in a meta-analysis of over 5000 patients. They were superior to both balloon angioplasty and bare metal stents.10 Intracoronary imaging with OCT provides a detailed assessment of in-stent restenosis mechanisms,11,12 and thus it should be considered to investigate the cause of restenosis.9 In keeping with this, we chose to treat the patient with a DES and used intracoronary imaging to guide both procedures.
In this proof-of-concept procedure we have shown that patient-specific simulation is feasible to guide the treatment strategy of complex coronary artery disease. We used a realistic PCI simulator in which we included patient-specific coronary anatomy in order to plan and practice a complex procedure beforehand, predict possible complications and gain confidence. In this way, the operator was able to use standard diagnosis and PCI tools in the catheterization laboratory environment, enabling testing and training for several endovascular skills. Furthermore, the final results of PCI can be seen using angiography, intravascular imaging and by directly inspecting the 3D anatomic model. Using this approach, we treated a critical ostial Cx stent restenosis with a simulation of the procedure the day before the actual PCI, using similar tools and achieving similar results. We hypothesize that complex procedures will be guided by patient-specific training in the future, if these encouraging preliminary results are replicated and tested in clinical trials. However, we must bear in mind that some complications are difficult to predict, such as edge dissection, iatrogenic coronary thrombosis, spasm, no-reflow, perforation or retained material due to calcification. In addition, simulated vessel wall properties are critical for procedure replication. This simple model has limitations in simulating calcifications/previous stents in the vessel wall, which might lead to differing balloon behavior when comparing the real and simulated procedures.
There are still some challenges in implementing patient-specific simulation using medical 3D printing. First, 3D-model accuracy heavily depends on image quality and precise segmentation to generate final volume. Secondly, it is challenging to replicate the complexity of coronary structure, namely with uneven calcification or previous stent implantation. Finally, this technology is demanding and requires multidisciplinary expertise, including cardiovascular imaging, software processing, 3D printing and materials selection.
ConclusionThis case illustrates the feasibility and accuracy of a 3D patient-specific simulation-guided treatment strategy for coronary PCI. This technology may improve operators’ experience, increase their confidence and offer a trustworthy anatomic model to test devices and complex intervention techniques.
Conflicts of interestManuel Oliveira Santos, Eduardo Oliveira Santos and João Silva Marques have created and developed the custom interventional cardiology simulator SimulHeart®.
The authors have no other conflicts of interest to declare.
The authors acknowledge the contribution of Paulo Carvalho for technical support with the fluoroscopy equipment for the simulated procedure.