Left ventricular noncompaction (LVNC) is characterized by left ventricular (LV) hypertrabeculations and is associated with heart failure, arrhythmias and embolism. We report the case of a 67-year-old LVNC patient, under oral anticoagulation (OAC) therapy for apical thrombosis. After she discontinued OAC, the thrombus involved almost the whole of the left ventricle; in a few months her condition worsened, requiring hospitalization, and despite heparin infusion she experienced myocardial infarction (MI), caused by embolic occlusion of the left anterior descending artery. Although infrequent as a complication of LVNC, and usually attributable to microvascular dysfunction, in this case MI seems due to coronary thromboembolism from dislodged thrombotic material in the left ventricle.
A não compactação ventricular esquerda (NCVE) é caracterizada por hipertrabeculações ventriculares esquerdas (VE) e está associada à insuficiência cardíaca, arritmias e embolias. Divulgamos o caso de uma doente de 67 anos com NCVE e em terapêutica de anticoagulação oral (ACO) por trombose apical. Como descontinuou a anticoagulação oral o trombo envolveu quase todo o VE; em poucos meses a sua situação piorou necessitando internamento e – apesar da infusão com heparina – sofreu um enfarte do miocárdio (EM), causado por oclusão embólica da DAE. Embora seja pouco frequente tal como a complicação por NCVE e seja geralmente atribuível à disfunção microvascular, o EM parece ser, neste caso, devido ao tromboembolismo coronário a partir do trombo do VE.
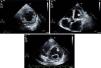
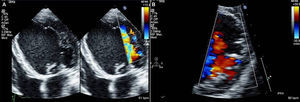
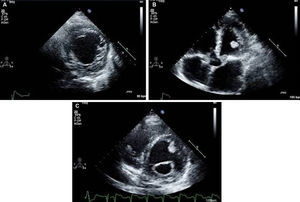
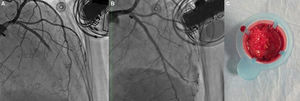
Isolated left ventricular noncompaction (LVNC) is a form of cardiomyopathy resulting from persistence of fetal trabeculations and intertrabecular recesses within ventricular myocardium. The clinical features associated with LVNC consist of left ventricular (LV) systolic dysfunction, arrhythmias, and thromboembolic events. We report the case of a 67-year-old woman admitted to the emergency department with acute aphasia, dyspnea, and peripheral edema associated with recent-onset paroxysmal atrial fibrillation (AF) with high ventricular rate. After anticoagulation with unfractionated heparin (UFH), she was converted to stable sinus rhythm with intravenous (IV) amiodarone. She was then admitted to the intensive coronary care unit (ICCU) and treated with IV inotropes and diuretics, resulting in prompt recovery from aphasia and improvement in congestion. The echocardiogram showed a markedly dilated left ventricle with hypertrabeculation of the apex and of the inferior-inferolateral segments (noncompacted/compacted ratio 2:1), severely reduced ejection fraction (EF) (22%), and an apical thrombus. No significant carotid artery disease was found on Doppler echocardiography. She underwent implantation of an implantable cardioverter-defibrillator and was discharged in NYHA class II, under standard heart failure therapy including oral anticoagulation (OAC), with no evidence of thrombosis (Figure 1). Twelve months later, due to a major depressive episode, the patient failed to attend the scheduled heart failure clinic (HFC) follow-up and discontinued OAC. Due to recurrent dyspnea and fatigue she presented to the HFC, where an echocardiogram showed a massive LV thrombosis (Figure 2), so she was admitted to the ICCU and IV UFH was started. After two days the patient complained of chest pain; as the ECG showed marked ST segment elevation in V3-V6 she was referred to the catheterization lab. Coronary angiography revealed a thrombotic occlusion of the mid segment of the left anterior descending artery (Figure 3A); the clot was aspirated and no significant coronary artery disease (CAD) was found (Figure 3B and C). A marked increase in plasma troponin I was observed, confirming the diagnosis of acute embolic myocardial infarction (MI). Her EF fell to 15% and after two days she became hypotensive despite intra-aortic balloon pump and inotropic support, with cardiogenic shock and acute kidney failure. She was considered for a left ventricular assist device, but sepsis and multiorgan failure occurred, and death followed 25 days later.
(A) Parasternal short-axis view of the mid segments. Two-layered structure of the thickened myocardium, with deep trabecular recesses in inferior, inferolateral and anterolateral segments; (B) apical 5-chamber view showing a floating thrombotic mass; (C) parasternal short-axis view showing thrombotic masses in the basal and mid segments of the anterior wall of the left ventricle.
(A) Left coronary angiogram showing a thrombotic total occlusion in the mid segment of the left anterior descending artery (LAD); (B) left coronary angiogram following percutaneous coronary intervention showing no significant stenoses; (C) thrombotic material retrieved from the LAD by thrombus aspiration.
LVNC is associated with HF, arrhythmias and embolism. Cardioembolic events are not uncommon, as trabecular recesses and depressed systolic function predispose to thrombosis, but presentation as an acute coronary syndrome (ACS) is rather unexpected.1 Previous observations suggested that in patients with reported myocardial infarction and LVNC, ischemia is mainly related to CAD and does not appear to be relevant to LVNC.2 However, in the small number of reported cases of ACS in LVNC patients with no evidence of coronary stenosis or LV thrombosis, myocardial infarction was described as a consequence of microvascular dysfunction, as LVNC patients exhibit decreased coronary flow reserve in both compacted and noncompacted LV segments3 and subendocardial perfusion defects despite normal coronary arteries.4 In a few of them embolism from thrombosis of the LV chamber was suggested, but with no reported evidence of intracavitary thrombus.5 In our patient, significant angiographic CAD was absent and the evidence of LV thrombosis itself strongly supports embolism as the most likely etiology. Although MI as an embolic complication is relatively infrequent, stroke and/or embolism occur in at least 15% of LVNC patients, mostly in those with advanced HF and AF,6 which implies that OAC is worth starting in the presence of predisposing factors. Nevertheless, in the absence of such conditions, cardioembolic events are rare, and stroke and/or embolism may also have an atherosclerotic cause. Hence, the embolic risk in LVNC patients with systolic dysfunction in sinus rhythm is largely unknown and OAC is mainly an individual therapeutic choice, mandatory in those with evidence of LV thrombosis, or a prudent option in primary prevention for those at the highest risk of embolization.7 In our case OAC was initially prescribed because of a previous transient ischemic attack with LV thrombosis, and paroxysmal AF.
In conclusion, this case suggests that, although infrequent, ACS is a possible manifestation of LVNC-related embolism, and so an embolic etiology should be kept in mind in differential diagnosis between atherosclerosis in LVNC patients presenting with MI. Careful attention should be paid to embolic risk stratification and the need for OAC in such patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.