Oxymetazoline is an alpha-1 adrenergic receptor agonist that is commonly used for nasal decongestion and is readily available without a prescription. We report the case of a 64-year-old woman who developed prolonged chest pain associated with elevation of cardiac biomarkers after using oxymetazoline.
A oximetazolina é um agonista alfa-1-adrenérgico, que é comummente utilizada para o descongestionamento nasal. Relatamos o caso clínico de uma doente de 64 anos de idade com dor no peito prolongada após o uso de oximetazolina, associada à elevação de biomarcadores cardíacos.
Oxymetazoline nasal spray is a commonly used long-acting topical nasal spray containing 0.05% oxymetazoline, an alpha-1 adrenergic agonist, and is readily available over the counter for nasal congestion. It is generally considered to be a safe drug, with no reported associated cases of myocardial infarction. In this report, we describe a 64-year-old African American woman who had prolonged chest pain associated with elevation of cardiac biomarkers after using oxymetazoline.
Case reportA 64-year-old African American woman with a history of diabetes mellitus and hypertension presented to the emergency room with chest pain. She had been seen by her primary physician two days previously for nasal congestion, after which she was prescribed oxymetazoline nasal spray, azithromycin and mometasone nasal spray. One day prior to admission, she developed a sharp substernal chest pain approximately one minute after using the oxymetazoline nasal spray. The pain lasted for fifteen minutes and resolved spontaneously. On the day of admission, the patient had another episode of chest pain which also occurred around a minute after administering two squirts of the oxymetazoline nasal spray. This time the pain was retrosternal, sharp in nature, 9/10 in severity, radiating to her left arm, associated with nausea and one episode of vomiting, and was relieved by sublingual nitroglycerine and aspirin in the emergency room. She is a former smoker and has no history of illicit drug use or of vasospastic disorders such as migraine or Raynaud's phenomenon.
Her home medications included metformin 500 mg twice daily, nateglinide 60 mg twice daily, atorvastatin 10 mg daily, triamterine 37.5 mg daily and losartan 50 mg daily. She had no prior history of heart disease or of similar complaints. She had also never used oxymetazoline nasal spray before.
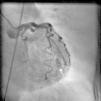
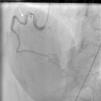
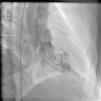
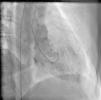
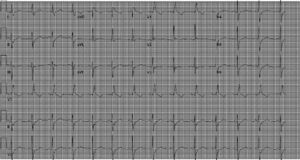
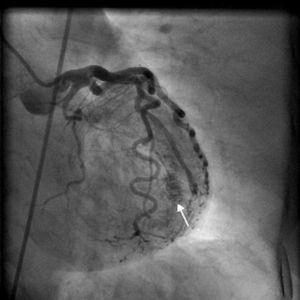
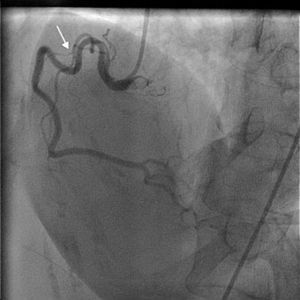
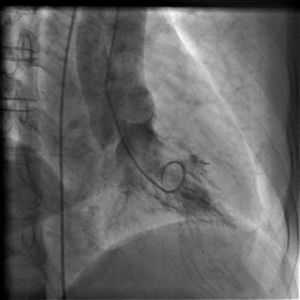
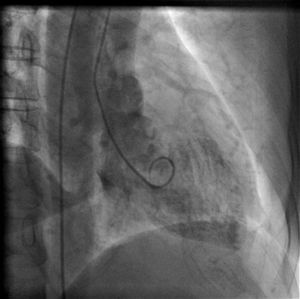
On arrival in the emergency room, the patient was found to be hypertensive with blood pressure of 194/82 mmHg and was afebrile with a heart rate of 83 beats per minute and respiratory rate of 20 cycles per minute. The rest of the physical exam was unremarkable, with clear lungs on auscultation of the chest, normal heart sounds and no murmurs, rubs or gallops on cardiac auscultation. She had no pedal edema and peripheral pulses were normal. The ECG at admission showed right bundle branch block at a rate of 81/min (Figure 1). Laboratory tests showed normal hemoglobin (11.4 g/dl), creatinine 1 mg/dl and potassium 3.3 mmol/l. Cardiac enzymes showed troponin 1.81 ng/ml (reference: 0.00–0.05 ng/ml) and CKMB 5.2 ng/ml (reference: 0.5–3.6 ng/ml). A portable chest X-ray was also unremarkable. Based on typical history and elevated cardiac markers, the patient was diagnosed with non-ST-elevation myocardial infarction and was started on aspirin, clopidogrel, metoprolol, lisinopril and heparin. She underwent left heart catheterization, which showed normal coronary arteries (Figures 2 and 3) and a small coronary-cameral fistula (CCF). Ejection fraction was 75% on left ventriculography (Figures 4 and 5). Echocardiography showed no source of emboli or valvular disease. Anemia, arrhythmias or hypotension were also not evident in our patient and type 2 myocardial infarction with decreased oxygen supply and/or increased demand after use of oxymetazoline was considered as the diagnosis.
Her hospital course was uneventful and she was discharged in a stable medical condition on daily aspirin 81 mg, as needed, sublingual nitroglycerin and her home medications. She was instructed to avoid using the oxymetazoline nasal spray in the future. At six-month follow-up she was free of symptoms.
DiscussionOxymetazoline, an imidazoline derivative, is an established long-acting nasal decongestant. Our patient's symptoms were resolved by the time she was seen in the hospital; her ECG and echocardiogram were normal but elevated troponin was consistent with ischemic episodes which occurred immediately after using oxymetazoline. Oxymetazoline use in adults has been associated with bradycardia, hypotension, near-syncope in a 73-year-old male,1 recurrent ventricular tachycardia in another adult2 and severe hypertension and cardiac arrest in a 2-year-old patient.3 However, there were no reported cases of angina or myocardial infarction caused by oxymetazoline in our search of the literature, in contrast to the use of other topical nasal decongestants such as phenylephrine and cocaine, which are more commonly associated with adverse events including myocardial ischemia.4,5 In fact, in a prospective study in a pediatric population, 0.05% oxymetazoline applied to the nasal mucosa before endoscopic sinus surgery appeared to affect heart rate and blood pressure less than 0.25% phenylephrine hydrochloride at 5 and 10 minutes after application.6
However, oxymetazoline is still a potent alpha-1 adrenergic agonist and has the potential to cause systemic vasoconstriction and hypertension. This can cause myocardial oxygen supply/demand mismatch and can precipitate angina or myocardial infarction, especially in patients with impaired coronary circulation. Our patient had risk factors for coronary artery disease; however she had no prior history of angina and no significant coronary artery disease on coronary angiography, which further strengthens the hypothesis of vasospasm and/or myocardial oxygen supply/demand mismatch as the mechanism for angina and myocardial injury in this case.
Our patient was discharged home on aspirin and sublingual nitroglycerin as needed. There are limited data on antiplatelet use in type 2 myocardial infarction and/or coronary vasospasm, but it has been shown in an internal mammary artery model that vascular smooth muscle cells express P2Y12 receptors and that these mediate contractile function after stimulation with ADP.7
Thromboxane and 5-hydroxytryptamine also stimulate platelet aggregation and vasoconstriction.7 All of these contractile effects could play a role in the thrombogenic vasospasm that is seen in unstable angina or subarachnoid bleeding. Antiplatelet agents such as aspirin or P2Y12 inhibitors could potentially be used in certain cases where coronary artery spasm is suspected.
CCF is an anomalous connection between a coronary artery and a cardiac chamber. The small CCF seen in our patient was probably just an incidental finding without clinical implications in this context. Most CCFs are discovered incidentally during angiographic evaluation for coronary vascular disorders and are reported in 0.08%–0.3% of unselected patients undergoing diagnostic coronary angiography.8
Our case highlights a potential adverse effect of a drug that has not been previously reported. The use of topical nasal vasoconstrictors is widespread in the community and the incidence of reported side effects is negligible. A history of medication use in a patient with angina or myocardial infarction is often missed as the emphasis is mostly on elucidation of risk factors for coronary artery disease. However patients with multiple risk factors are most susceptible to adverse drug reactions. The importance of knowledge of drug pharmacology in recognizing potential adverse drug reactions cannot be overemphasized. We hope that this case report will increase awareness of a potentially life-threatening side effect of this commonly used medication in susceptible patients and will aid differential diagnosis and treatment.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.