Ischemic cardiomyopathy is the leading cause of heart failure. In patients with left ventricular (LV) dilatation, low ejection fraction, and transmural scar in an anteroseptal distribution, surgical ventricular reconstruction (SVR) is a treatment option.
We describe our first experience with the Less Invasive Ventricular Enhancement (LIVE) technique using the Revivent™ system (Bioventrix Inc., San Ramon, CA), in the treatment of a large anteroapical aneurysm.
A cardiomiopatia isquémica é a principal causa de insuficiência cardíaca. A cirurgia de reconstrução ventricular é uma opção terapêutica em doentes com dilatação e disfunção sistólica do ventrículo esquerdo e cicatriz de enfarte transmural da parede anterior e septo.
Os autores pretendem descrever a sua experiência inicial com uma técnica menos invasiva de reconstrução ventricular (Less Invasive Ventricular Enhancement [LIVE]) utilizando o sistema Revivent™ (Bioventrix Inc., San Ramon, CA, EUA), no tratamento de um volumoso aneurisma ântero-apical.
Surgical ventricular reconstruction (SVR) has become a surgical option for patients with severe ischemic heart failure. SVR effectively reduces LV volume and wall tension and is known to improve neurohormonal status.1–3 However, its impact on mortality, functional capacity and quality of life is still controversial.4
Recently, a new technique, Less Invasive Ventricular Enhancement (LIVE), has been developed using the Revivent™ Myocardial Anchoring System (Bioventrix Inc., San Ramon, CA). In this technique, plication and exclusion of the aneurysm is achieved using anchors which are implanted into the scarred portion of the heart, rendering the LV smaller. Conceptually, the final configuration in SVR can be achieved by placing these implants. However, Revivent™ achieves this outcome without an LV incision and thus without the need for cardiopulmonary bypass (CPB).
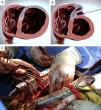
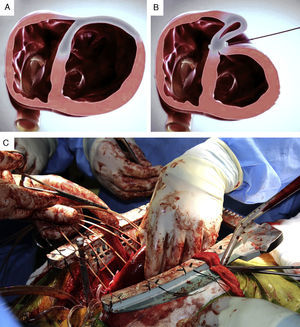
The system consists of four components: a catheter-based delivery system, a force gauge to control the anchors when apposing the LV walls, the implantable anchor elements, and a retrieval system (to remove the implants if necessary). The implantable components consist of polyester fabric-covered titanium anchor pairs: one hinged internal anchor that passes through a low-profile catheter to the right side of the septum and one locking external anchor deployed in an LV epicardial position (Figure 1A and B).
Case reportWe report the case of a 41-year-old Caucasian male evaluated in our center for severe ischemic cardiomyopathy. The patient had suffered a transmural anterior myocardial infarction six months earlier for which he sought no medical help, and was in NYHA class II despite optimal heart failure therapy for at least 90 days (160 days). His past history included smoking, hypertension, dyslipidemia and type II diabetes. He was evaluated after this event for post-infarction angina. Catheterization showed occlusion of the proximal left anterior descending coronary artery without additional lesions.
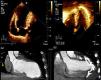
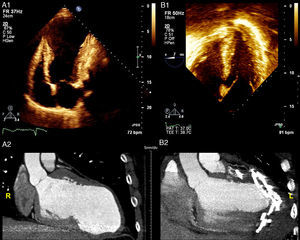
Transthoracic echocardiography (TTE) depicted a severely dilated LV with a large anteroapical aneurysm and severe LV dysfunction (Figure 2A1), which was also seen on the cardiac computed tomography scan (Figure 2A2). Mild mitral regurgitation was present. Due to the absence of tricuspid regurgitation, pulmonary artery systolic pressure could not be estimated.
(A1) Baseline transthoracic echocardiogram in 4-chamber view showing the large LV aneurysm and preserved basal segments; (A2) cardiac computed tomography (CT) scan before the procedure to assess the possibility of percutaneous treatment; (B1) intraoperative transesophageal echocardiogram showing normalization of ventricular shape and reduction of LV volume; (B2) cardiac CT scan post-procedure showing the anchor pairs and absence of contrast leakage between the excluded scar and the LV.
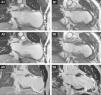
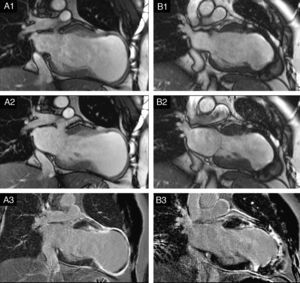
Cardiovascular magnetic resonance (CMR) confirmed these findings, with LV end-systolic volume index (LVESVI) of 223 ml/m2, LV end-diastolic volume index (LVEDVI) of 264 ml/m2 and ejection fraction (EF) of 0.15 (Figure 3A1 and A2). The apex and distal segments of all myocardial walls presented transmural (>75%) late gadolinium enhancement, indicating absence of myocardial viability (Figure 3A3). Apical thrombus was detected.
(A) Preoperative long-axis (2-chamber) cine cardiac magnetic resonance (CMR) obtained at end-diastole (A1) and end-systole (A2). Note the severe LV dilatation with a large anterior/apical aneurysm. The apex and distal segments of all walls had thin walls and were dyskinetic in cine studies; (A3) late gadolinium enhancement (LGE) CMR revealing transmural scar (>75%) in the distal segments of all walls and apex, and subendocardial scar in the mid segments of the anteroseptal and anterior walls. Apical thrombus was detected (necessitating oral anticoagulation before surgery); (B) six-month follow-up cine CMR at end-diastole (B1) and end-systole (B2) showing significant reduction in LV volumes. In cine studies the apex exhibits dyskinesia; (B3) in LGE CMR, the apex and mid and distal segments of the anterior and anteroseptal walls presented transmural scar. Note the signal void caused by the metal anchors (titanium).
The patient provided informed consent and was enrolled in the CONFIGURE-HF trial (phase II), a multicenter, prospective, single-armed study designed to establish the efficacy and safety of the new LIVE system for left ventricular volume reduction in patients with heart failure due to large anteroseptal scars. Major inclusion criteria are: (a) EF <0.35; (b) LVESVI >60 ml/m2 (preferably <120 ml/m2); (c) symptomatic heart failure (NYHA class II-IV) despite optimal medical therapy for at least 90 days; (d) age 18–75 years; (e) no infarction within three months of operation; (f) referral for ventricular reduction operation. Major exclusion criteria are: (a) need for concomitant mitral valve surgery; (b) the presence of intracardiac thrombus; (c) contraindication for warfarin therapy in the three months after surgery.5
In our patient, the presence of apical thrombus required four months of anticoagulation and repeated imaging showing resolution of the thrombus, for inclusion. His LVESVI >120 ml/m2 was not an impediment, since the basal segments and other LV segments had preserved contractility.
After sternotomy, extensive scarring of the anterior LV wall was evident on inspection. A total of seven anchor pairs were implanted under direct fluoroscopic and transesophageal echocardiography (TEE) guidance (Figure 1C).
The considerable size of the aneurysm rendered this case unique, as only one anchor was passed through the LV septum, and the others were placed entirely in an epicardial position. Also of note, an above-average number of anchors were necessary to achieve the final result.
The procedure lasted 209 minutes (skin to skin). No complications occurred. TEE at the end of the procedure showed normalization of ventricular shape, significant reduction of LV volumes (>50%) and slightly improved EF (Figure 2B1). The patient was discharged home on the 7th day post-procedure. On discharge, no leakage between the excluded scar and the LV was observed (Figure 2B2).
At the one-month follow-up visit, the patient was in NYHA class I and had resumed normal daily activities. Functional capacity assessed by the six-minute walk test improved significantly at six months (138 m improvement compared to baseline).
CMR at six months demonstrated significant LV volume reduction (LVESVI: 87 ml/m2, LVEDVI: 143 ml/m2) and improvement in EF (39%) (Figure 3B1 and B2).
DiscussionSVR has been demonstrated to be beneficial in selected patients.2,6–8 However, there is substantial debate over the superiority of SVR with coronary artery bypass grafting (CABG) compared to CABG alone.4 Recently, results from a post-hoc analysis of the Surgical Treatment for Ischemic Heart Failure (STICH) randomized trial demonstrated that there are subgroups of patients who can benefit from SVR.9 For example, a postoperative LVESVI of 70 ml/m2 or less after CABG plus SVR resulted in enhanced survival compared with CABG alone. Also, an analysis by Dor et al., in a patient cohort excluded from the STICH trial, demonstrated favorable results of SVR, indicating that is not possible to extrapolate the results of STICH to all this population.10
In high-risk patients, the apparently improved risk profile of the LIVE technique may be an advantage compared to other SVR procedures, as it does not require LV incision or CPB and is a potentially more rapid procedure. Furthermore, it has potential advantages regarding standard linear resection, as the septum is included in the repair. Early results of this technique were recently published and documented an effective volume reduction at one-year follow-up.5
As highlighted in this case report, in the LIVE technique the walls of the ventricle stay perfectly aligned and with no residual communication between the LV and the excluded cavity, reducing the risk of clots, a concern in this type of ‘closed’ procedure.5 Even so, patients are empirically anticoagulated for three months after the procedure.
In our patient the large size of the aneurysm and severely compromised LV systolic function did not preclude a favorable outcome. This could be explained by the preserved contractility of other LV segments and the relatively early treatment. Nevertheless, some concern was raised regarding long-term remodeling, with the six-month follow-up CMR showing incomplete exclusion of the septal scar and a dyskinetic apex (Figure 3B3).
The results of the CONFIGURE-HF trial and long follow-up data are required to determine the long-term safety and effectiveness of the technique.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to express our gratitude to Dr. Nuno Bettencourt and Dr. Nuno Ferreira of the Cardiovascular Imaging Department and to Dr. Rodolfo Pereira of the Department of Cardiothoracic Surgery.