Protein C deficiency is a coagulation cascade disorder often resulting in venous thromboembolic events but is also a possible contributor to arterial thrombosis. To date, approximately ten cases of myocardial infarction (MI) due to protein C deficiency have been reported in the literature. However, affirming this mechanism requires ruling out the most common causes of MI, i.e. the rupture or erosion of an atherosclerotic plaque. Intravascular imaging of coronary arteries can be of help to identify angiographically undetected atherosclerosis. We report a case of an ST-segment elevation myocardial infarction (STEMI) in a young man with apparent evidence of arterial thrombosis resulting from protein C deficiency and heterozygous factor Leiden mutation which was contradicted by intravascular imaging demonstrating atherosclerosis.
A deficiência de proteína C constitui uma alteração em cascata da coagulação, dá origem muitas vezes aos eventos tromboembólicos venosos e é também um contributo possível de trombose arterial. Aproximadamente dez casos de enfarte do miocárdio (EM) devidos à deficiência da proteína C foram referenciados na literatura. No entanto, a confirmação desse mecanismo requer a exclusão das etiologias mais comuns de EM, i.e. a rotura ou a erosão da placa aterosclerótica. A avaliação imagiológica intravascular das artérias coronárias poderia ser uma ajuda para identificar a aterosclerose não detetada por angiografia. Apresentamos o caso de um enfarte do miocárdio com elevação do segmento-ST (STEMI) que revela evidência aparente de trombose arterial devida à deficiência da proteína C e à mutação do fator heterozigótico Leiden que foi contestada através de avaliação imagiológica intravascular e demonstrou aterosclerose num homem novo.
Combined thrombophilia can be a cause of myocardial infarction, but patients with thrombophilia should be thoroughly investigated for atherosclerosis in the event of coronary thrombosis. Intravascular imaging can help identify atherosclerosis which has remained undetected by angiogram. This has an impact on patient management, as antiplatelet therapy becomes mandatory if atherosclerosis is detected. There are no recommendations at present for the treatment of acute coronary syndrome in patients with thrombophilia and atherosclerosis.
Case reportA 40-year-old Caucasian man with an asymptomatic heterozygous Protein C deficiency and a history of pulmonary embolism in first-degree relatives was admitted with a three-day history of repeated episodes of chest pain at rest lasting 15 to 30 minutes. He had no cardiovascular risk factors (obesity, dyslipidemia, smoking, diabetes, hypertension, familial history).
At admission, the patient was asymptomatic. He was hemodynamically stable with a blood pressure of 125/83 mmHg and a heart rate of 90 bpm. Physical examination was unremarkable. The initial ECG revealed anterior sequelae of ischemia with anterior ST-segment elevation. Initial troponin T concentration was 1538 pg/mL (normal <14 pg/mL) and renal function was normal (creatinine 65 μmol/L, normal 59-104 μmol/L). Further laboratory investigations found that complete blood count, blood chemistry and lipid profile were all within normal ranges. Troponin concentration was in the ascending phase (2156 pg/mL on day 1, 2869 pg/mL on day 2). The screening for antiphospholipid antibodies was negative. Antithrombin was 97% (normal 80-120%). Protein C concentration was abnormal, with a level of 61% (normal 70-120%) checked by chronometric and chromogenic methods. A heterozygous Factor V Leiden mutation was also found in addition to his protein C deficiency. Therefore, this patient had a combined thrombophilia.
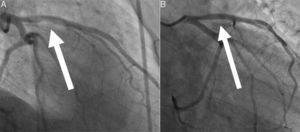
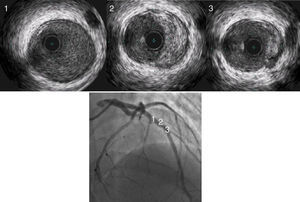
Transthoracic echocardiogram showed a depressed left ventricular ejection fraction of 34% with apical akinesia, no valve diseases, and no visible thrombi. Coronary angiography showed proximal and medial left anterior descending occlusions caused by a massive thrombus (Figure 1, panel A; moving angiograms in supplementary materials). Circumflex and right coronary arteries were normal. In view of the history of thrombophilia, coronary angioplasty was not initially performed and antithrombotic treatment combining anticoagulation with unfractionated heparin (monitored by anti-Xa activity, targeting 0.3 to 0.7 IU/mL) and dual antiplatelet therapy was initiated. A second coronary angiogram was performed after five days of antithrombotic treatment and showed complete regression of the thrombus with an underlying normal left anterior descending artery (Figure 1, panel B). However, an intravascular ultrasound study (IVUS) revealed an atheromatous plaque (Figure 2). Thus, we hypothesized that two mechanisms were in play: the rupture of an atheromatous plaque initiating an anterior myocardial infarction aggravated by his mixed coagulopathy (Protein C deficiency associated with Factor V Leiden mutation).
Protein C is a vitamin K-dependent glycoprotein that plays an important role in the regulation of blood clotting as a natural anticoagulant.1 Protein C deficiency is a known risk factor for venous thromboembolic events,2 but also a risk factor for arterial thrombosis, in particular myocardial infarction. Indeed, about twenty case reports and a large study3 have been published in the past few years regarding patients with heterozygous Protein C deficiency responsible for ischemic cerebral stroke or myocardial infarction. Isolated heterozygous Factor V Leiden mutation usually does not induce myocardial infraction4 but there have been a few case reports on myocardial infarction provoked by a combined thrombophilia.5
This case could be included in a larger entity called “Myocardial infarction with non-obstructive coronary arteries (MINOCA)”. As Pasupathy et al. said in their review paper,6 “this diagnosis is made in a patient presenting with diagnostic features of an acute myocardial infarction, in whom angiography does not show obstructive coronary artery disease, and there is no immediately apparent cause for the presentation.” It could be considered as a “working diagnosis”. In fact, it does not consist of a definitive diagnosis but it does allow us to classify a clinical entity in order to better manage investigation of the “real diagnosis”. A recent meta-analysis7 showed an association between MINOCA and thrombophilia. Indeed, Protein C deficiency has a prevalence of 0.1% to 1% in the general population, reaching 2.6% in patients with MINOCA,6 whereas factor V Leiden mutation has a prevalence of 3% to 7% in Western countries, but affects 12% of MINOCA patients.
Atherosclerosis is the most common cause of myocardial infarction. As recommended in European guidelines, when coronary angiography is normal, intravascular imaging can be used to detect small atherosclerosis plaques (class IIbB recommendation).8 In our patient, to improve diagnostic accuracy, we performed an IVUS which revealed an atherosclerotic plaque in an angiographically normal artery. We then hypothesized, even though a lack of scientific evidence means that this entity remains under debate, that the patient had a STEMI for the following reasons: rupture of an atherosclerotic plaque initiating thrombus genesis, amplified by the thrombophilia.
This case demonstrates: (i) that IVUS is of great value to more accurately rule in/out atherosclerosis when a coronary thrombosis is suspected in patients with clotting disorders; and (ii) the necessity of establishing recommendations to manage this situation and properly assess this rare diagnosis, as there is a lack of consensus in the current literature regarding the management of such patients.
AuthorshipArnaud Hubert, Raphael Martins and Vincent Auffret: Writing of the case report, clinical care.
Guillaume Leurent and Marc Bedossa: Performing and interpretation of angiography/intravascular ultrasounds.
Pierre Guéret: Interpretation of coagulation disorders.
Conflicts of interestThe authors have no conflicts of interest to declare.