We report the case of a 53-year-old male patient with a medical history significant for paroxysmal atrial fibrillation, migraines with visual aura and non-obstructive coronary artery disease, who sustained a non-ST-elevation myocardial infarction a few hours after taking eletriptan as abortive therapy for migraine headaches. We believe this case implies a causal association between eletriptan and myocardial infarction, considering the timing of both drug intake and symptom onset. To the best of our knowledge this is the first reported myocardial infarction attributable to eletriptan overdose in a patient without obstructive coronary artery disease.
Relatamos o caso de um doente de 53 anos de idade com uma história médica passada de fibrilhação auricular, enxaqueca com aura visual e doença coronária não obstrutiva que culminou em enfarte agudo do miocárdio poucas horas após toma de eletriptano como terapia abortiva para enxaqueca com aura. Dada a relação temporal entre a ingestão do eletriptano e o início dos síntomas, os autores defendem um nexo de causualidade entre a sobredosagem de eletriptano e a síndrome coronária aguda. Este é o primeiro caso descrito de enfarte agudo do miocárdio após sobredosagem de eletriptano num doente sem doença coronária obstrutiva.
Triptans are agonists of the 5-HT1B and 5-HT1D receptors known to induce relief of migraine symptoms by causing cranial vasoconstriction, acting on postsynaptic receptors of vascular smooth muscle cells. Both 5-HT1B and 5-HT2A receptors can trigger coronary artery spasm but only 5-HT1B receptors appear to mediate coronary vasospasm of patients treated with triptans.
Eletriptan is a highly selective serotonin 5-HT (1B/1D) receptor agonist that is effective in the acute treatment of migraine.1 The drug is rapidly absorbed when orally administered, has good bioavailability (50% compared to 14% for sumatriptan) and a long half-life, which enhances its ability to prevent recurrent headaches.2
Serious adverse cardiac events including acute myocardial infarction due to coronary vasospasm, arrhythmias and death have been reported after the administration of 5-HT1 agonists.3,4
In the literature, symptoms of chest pain, neck tightness and chest pressure have been widely described in patients taking 5-HT1 agonists, but few serious cardiovascular events such as myocardial infarction have been reported.5–11
Case reportA 53-year-old male patient with a medical history significant for hyperlipidemia, paroxysmal atrial fibrillation and migraines with visual aura (one or two attacks a month) presented to the emergency department (ED) complaining of chest pain. On the previous afternoon, the patient experienced a typical migraine attack and took 40 mg of eletriptan, which partially relieved his headache.
On the following morning, given the persistence of his symptoms, he decided to take two 40-mg eletriptan tablets (maximum recommended single dose 40 mg or 80 mg in a 24-hour period). Four hours later, while driving home from work, he developed sudden-onset midsternal chest pain, nonradiating and severe in intensity, which led him to seek medical attention. He denied having similar symptoms in the past. He was a lifelong nonsmoker and exercised regularly. He had no personal history of diabetes or hypertension. There was also no family history of premature coronary artery disease. On admission he was still in pain. The respiratory and cardiovascular examinations were unremarkable. Neurological examination revealed no neurological deficits. The first electrocardiogram (ECG) obtained in the ED showed sinus bradycardia with a heart rate of 55 bpm, without ST-T wave changes. Initial and peak troponin were 1.18 ng/ml and 8.36 ng/ml, respectively.
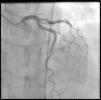
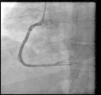
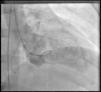
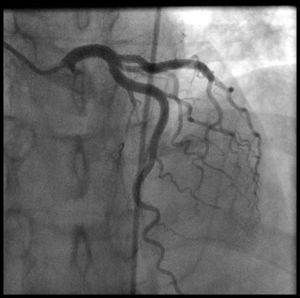
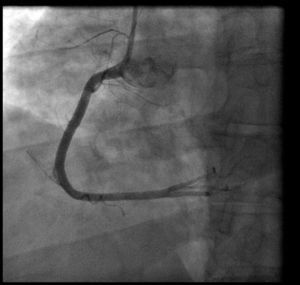
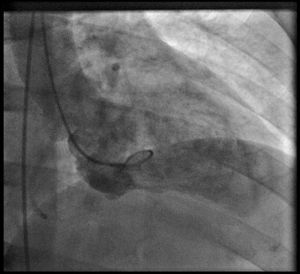
Coronary angiography (Figures 1 and 2) was performed 12 hours after his presentation and showed normal coronary arteries. Left ventriculography (Figure 3) revealed mild posterobasal hypokinesis. Left ventricular ejection fraction was mildly decreased.
DiscussionTo the best of our knowledge this is the first reported myocardial infarction attributable to eletriptan overdose in a patient without coronary artery disease.
Our patient presented to the ED complaining of chest pain associated with elevated cardiac biomarkers four hours after taking eletriptan. He was diagnosed with normal coronary arteries by coronary angiography 12 hours after admission. We believe this case implies a causal association between eletriptan and myocardial infarction, considering the timing of both drug intake and symptom onset. It is important to recall that the maximum recommended single dose of eletriptan is 40 mg or 80 mg in a 24-hour period, so our patient clearly overdosed on this drug.
The vast majority of reported triptan-related coronary events have occurred after intake of sumatriptan, zolmitriptan11,12 or tegaserod.10
Eletriptan is a potent 5HT1D/1B receptor agonist which in animal models induces coronary constriction at a dose four times higher than sumatriptan.13 Because of its higher selectivity for non-coronary vascular beds, eletriptan is considered to be the agonist of choice in patients with cardiovascular risk factors but no overt coronary artery disease.13
Muir et al.13 assessed the effects of intravenous eletriptan on the systemic, pulmonary and coronary circulation in patients without coronary artery disease undergoing cardiac catheterization. One patient experienced marked segmental right coronary artery constriction during drug infusion. Although this episode was associated with chest pain, no electrocardiographic abnormalities were detected, as in our patient.13,14
In the same study the authors claimed the chest pain could have resulted from catheter irritation, however we believe such an event occurring in the setting of an infusion of a well-known coronary vasoconstrictor drug is worrying and its potential to cause coronary vasospasm should not be disregarded.
It is important to identify patients at risk for dangerous cardiovascular events. Various screening tests, including electrocardiogram, nuclear stress test and stress echocardiography, have been proposed to assess cardiac risk prior to starting triptan therapy. However, no definite test has been standardized since the usefulness of assessing cardiovascular risk in asymptomatic patients with low pre-test probability of vasospastic phenomena is probably very limited.14 Our case also highlights the importance of educating patients not to exceed the prescribed dose as well as counseling them regarding early identification of angina symptoms if on triptans.
A new onset of chest pain in the setting of triptan use must alert patients to seek immediate medical attention and dissuade them from continuing to take increasing doses of triptans.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.