Percutaneous mitral valvuloplasty (PMV) is an effective treatment option for mitral stenosis (MS), but its success is assessed on the basis of clinical and echocardiographic outcomes in studies with relatively short follow-up. We aimed to characterize a cohort of patients undergoing PMV with long-term follow-up and to determine independent predictors of post-PMV mitral re-intervention and event-free survival.
MethodsWe studied 91 consecutive patients with MS who underwent PMV with a median clinical follow-up duration of 99 months. Two endpoints were considered: post-PMV mitral re-intervention (PMV or mitral surgery) and a composite clinical events endpoint including cardiovascular death, mitral valve re-intervention and hospital admission due to decompensated heart failure. We compared patients who required post-PMV mitral re-intervention with those who did not during follow-up.
ResultsThe study population included 83.5% females and mean age was 48.9±13.9 years. The 1-, 3-, 5-, 7- and 9-year rates of clinical event-free survival were 93.0±2.8%, 86.0±3.9%, 81.0±4.4%, 70.6±5.6%, and 68.4±5.8%, respectively. The 1-, 3-, 5-, 7- and 9-year rates of mitral re-intervention-free survival were 98.8±1.2%, 97.5±1.7%, 92.1±3.1%, 85.5±4.5%, and 85.5±4.5%, respectively. The median time to mitral re-intervention was 6.2 years. Patients who required mitral re-intervention during follow-up were younger (43.3 vs. 51.2 years, p=0.04) and had higher pre- and post-PMV mitral gradient (14.9 vs. 11.5mmHg, p=0.02 and 6.4 vs. 2.1mmHg, p<0.001) and higher post-PMV mean pulmonary artery pressure (mPAP) (30.0 vs. 23.2mmHg, p=0.01). In a Cox proportional hazards model, mPAP ≥25mmHg was the sole predictor of both mitral re-intervention (HR 5.639 [1.246–25.528], p=0.025) and clinical events (HR 3.622 [1.070–12.260], p=0.039).
ConclusionIn our population, immediate post-PMV mPAP was the sole predictor of post-PMV mitral intervention. These findings may help identify patients in need of closer post-PMV follow-up.
A valvuloplastia mitral percutânea (PMV) é uma opção eficaz para o tratamento da estenose mitral. Contudo, os resultados deste procedimento são baseados em estudos clínicos e ecocardiográficos com tempos de seguimento relativamente curtos. Os objetivos deste estudo são caracterizar uma população de doentes submetidos a PMV, realizar um seguimento clínico de longa duração e determinar os preditores independentes de reintervenção mitral após PMV e de sobrevida livre de eventos.
MétodosFoi estudada uma população de 91 doentes com estenose mitral tratada por PMV e realizou-se um seguimento clínico com a duração média de 99 meses. Foram pré-definidos dois endpoints: reintervenção mitral pós-PMV (PMV ou cirurgia mitral) e um endpoint clínico combinado que incluiu morte de causa cardiovascular, reintervenção mitral e internamento hospitalar por insuficiência cardíaca descompensada. Foi realizado um estudo comparativo entre os doentes que necessitaram de reintervenção mitral após PMV e os que não foram reintervencionados durante o tempo de seguimento clínico.
ResultadosA população estudada incluiu 83,5% de mulheres e a idade média foi de 48,9 ± 13,9 anos. As taxas de sobrevida livre de eventos aos 1, 3, 5, 7 e 9 anos foram de 93,0 ± 2,8%; 86,0 ± 3,9%; 81,0 ± 4,4%; 70,6 ± 5,6% e 68,4 ± 5,8%, respetivamente. As taxas de sobrevida sem reintervenção mitral aos 1, 3, 5, 7 e 9 anos foram de 98,8 ± 1,2%; 97,5 ± 1,7%; 92,1 ± 3,1%; 85,5 ± 4,5% e 85,5 ± 4,5%, respectivamente. O tempo mediano até à reintervenção mitral foi de 6,2 anos. Os doentes que necessitaram de reintervenção mitral durante o seguimento clínico eram mais novos (43,3 vs. 51,2 anos, p = 0,04), tinham gradientes transmitrais pré e pós-PMV mais elevados (14,9 vs. 11,5mmHg, p = 0,02 e 6,4 vs. 2,1mmHg, p < 0,001) e uma pressão média na artéria pulmonar (mPAP) pós-PMV mais elevada (30,0 vs. 23,2mmHg, p = 0,01). No modelo de regressão logística de Cox a mPAP ≥25mmHg foi o único preditor de reintervenção mitral (HR 5,639 [1,2-25,5], p=0,025) e de eventos clínicos (HR 3,6 [1,1-12,3], p=0,039).
ConclusãoNa população estudada, a mPAP medida imediatamente após a valvuloplastia mitral foi o único preditor de reintervenção mitral. Os resultados deste estudo podem contribuir para a identificação dos doentes que beneficiam de um seguimento pós-PMV mais frequente.
Percutaneous mitral valvuloplasty (PMV) is an effective treatment option for hemodynamically significant mitral stenosis (MS) and has become the procedure of choice in patients with suitable valve anatomy.1–5 However, the procedural success of PMV is assessed on the basis of clinical and echocardiographic outcomes in studies with relatively short follow-up (<5 years).2–4,6,7 Restenosis is the main mechanism of deterioration following an initially successful PMV3 and its development is time-dependent,3,8 reflecting the wide range of reported restenosis incidence (4–39%) according to different follow-up durations.2,3,9–11 Cardiac surgery may still be required for patients with post-procedural restenosis of the mitral orifice or increasing severity of mitral regurgitation (MR).6 In this study, we sought to characterize a cohort of patients undergoing PMV at a single institution and to perform a long-term clinical follow-up to assess the predictors of post-PMV mitral intervention and clinical event-free survival.
MethodsStudy populationThis single-center study included 91 consecutive patients with MS who underwent PMV between March 1992 and June 2010 in our institution. The indications for PMV were as follows: symptomatic patients with moderate or severe MS with favorable valve morphology; asymptomatic patients with moderate to severe MS and pulmonary hypertension (pulmonary artery systolic pressure >50mmHg at rest) with favorable valve morphology; and symptomatic patients with moderate or severe MS with unfavorable valve morphology but not candidates or at high risk for surgery. Determination of favorable valve morphology was based on echocardiographic evaluation. Data was collected prospectively and stored in an electronic database. Demographic, clinical and laboratory variables included age, gender, New York Heart Association (NYHA) functional class at presentation, presence of atrial fibrillation, prior surgical commissurotomy and echocardiographic score. Procedure-related variables included interventional technique, effective balloon dilating area normalized by body surface area, and pre- and post-PMV hemodynamic parameters (ventricular, atrial and pulmonary artery pressures, mean mitral gradient [MG]), cardiac output and calculated mitral valve area [MVA]. Procedural success was defined as MVA≥1.5cm2 and MR≤2/4, as used by Song et al.12 Written informed consent was obtained from all patients prior to PMV.
Echocardiographic evaluationComprehensive two-dimensional and color Doppler transthoracic echocardiogram was performed before PMV. The morphologic features of the mitral valve were categorized using the Wilkins echo score13 and the total echocardiographic score was obtained by adding the scores for leaflet mobility, thickness, calcification, and subvalvular lesions. MVA was measured by direct planimetry in parasternal short-axis view, and continuous wave Doppler was used to calculate peak pressure gradient of tricuspid regurgitation. Mitral and tricuspid regurgitation were graded from 0 to 4+, depending on the spatial extent of the color flow jet area expressed as a percentage of the left or right atrial area. Patients were screened for left atrial thrombus with a two-dimensional transesophageal echocardiogram in the 24h preceding the procedure. If thrombus was detected, the patient was not a candidate for PMV. Transthoracic echocardiographic measurement of MVA and quantification of MR were repeated one day after PMV.
Percutaneous mitral valvuloplastyAfter cardiac catheterization confirmed severe MS without significant MR, experienced physicians performed PMV using a percutaneous transseptal antegrade approach and the Inoue balloon technique, while monitoring conventional hemodynamic parameters. Balloon size was chosen to obtain an effective balloon dilatation area/body surface area of approximately 4cm2/m2, and one-step dilatation was performed. Maximum balloon size was determined by the following formula: patient's height (cm)/10+10. MVA was calculated using the Gorlin formula.14 Left ventriculography was performed to assess the presence and severity of MR, and Sellers’ classification was applied.15
Clinical follow-upClinical follow-up with a median duration of 99 months (interquartile range [IQR] 63–176) was performed for 85/91 (93.4%) patients. Clinical data was collected during patients’ visits to the outpatient clinic or by telephone interview. When required, primary care physicians and referring cardiologists were contacted and medical records were reviewed to obtain additional information. Surgical notes were obtained on all patients who underwent surgery after PMV. Two predefined study endpoints were assessed: (1) post-PMV mitral re-intervention (PMV or mitral surgery) and (2) a composite clinical events endpoint including cardiovascular death, mitral valve re-intervention and hospital admission due to decompensated heart failure. The population was divided into two groups according to need for post-PMV mitral re-intervention. Patients who underwent mitral surgery immediately after PMV due to iatrogenic severe MR were excluded from the analysis (n=3).
Statistical analysisContinuous variables were expressed as mean±SD. Median and IQR were used if the distribution was not normal, assessed by the Kolmogorov–Smirnov test. The Student's unpaired t-test for normal variables and the Mann–Whitney U test for non-normal variables were used for comparisons between groups. The paired-sample t-test was used to compare pre- and post-PMV variables. Categorical variables were presented as percentages, and were compared using the chi-square or Fisher's exact test. All variables were tested independently in a univariate logistic regression model, and those that achieved a significance level of p<0.10 were incorporated in a Cox proportional hazards regression model for both predefined endpoints. Receiver operating curves were calculated to determine the best cut-off values. Variables associated with a p value <0.05 were retained in the final model. Kaplan–Meier estimates were used to determine event-free survival. Comparison between groups was performed using the log rank test. Statistical analyses were performed with SPSS (13.0 for Windows, SPSS Inc., Chicago, IL, USA).
ResultsThe study population included 91 consecutive patients. Baseline clinical, echocardiographic and hemodynamic characteristics were compared in patients who required post-PMV mitral re-intervention and those that did not are summarized in Tables 1 and 2. All patients had an echo score under 8, reflecting the selected nature of this population.
Baseline clinical and echocardiographic characteristics of patients who did and did not require post-PMV mitral re-intervention.
| Total (n=82) | No post-PMV mitral re-intervention (n=64) | Post-PMV mitral re-intervention (n=18) | p | |
| Age (years) | 48.9±13.9 | 51.2±13.8 | 43.3±12.8 | 0.040 |
| Female gender | 84.1% | 83.6% | 86.7% | 0.767 |
| Body mass index (kg/m2) | 25.2±3.5 | 25.5±3.4 | 23.6±2.7 | 0.050 |
| Atrial fibrillation | 29.6% | 31.8% | 20.0% | 0.366 |
| Echo score | 6 (5–7) | 6 (5–7) | 6 (5–6) | 0.360a |
| Prior commissurotomy | 3.7% | 3.0% | 6.7% | 0.492 |
| MVA (cm2) | 1.1±0.2 | 1.1±0.2 | 1.0±0.2 | 0.370 |
| NYHA III/IV | 53.2% | 56.3% | 38.5% | 0.241 |
MVA: mitral valve area; NYHA: New York Heart Association; PMV: percutaneous mitral valvuloplasty. Results are expressed as mean±SD or median (interquartile range), as appropriate.
Hemodynamic findings in patients who did and did not require post-PMV mitral re-intervention.
| Hemodynamic data | Total (n=82) | No mitral re-intervention (n=64) | Mitral re-intervention (n=18) | p |
| MVA (cm2) (Gorlin) | ||||
| Pre-PMV | 1.0±0.3 | 1.0±0.3 | 0.9±0.3 | 0.160 |
| Post-PMV | 2.3±0.4 | 2.3±0.5 | 2.3±0.3 | 0.790 |
| Mean MG | ||||
| Pre-PMV | 12.3±5.2 | 11.5±5.0 | 14.9±5.1 | 0.020 |
| Post-PMV | 3.0±3.8 | 2.1±2.2 | 6.4±6.8 | <0.001 |
| RA pressure | ||||
| Pre-PMV | 7.8±4.5 | 7.6±4.8 | 8.9±3.4 | 0.380 |
| Post-PMV | 9.0±4.7 | 9.3±5.0 | 7.8±4.1 | 0.420 |
| Mean RV pressure | ||||
| Pre-PMV | 8.6±4.3 | 8.5±4.4 | 9.0±4.5 | 0.780 |
| Post-PMV | 9.9±5.4 | 10.0±5.7 | 9.7±4.4 | 0.880 |
| Mean PA pressure | ||||
| Pre-PMV | 32.0±12.7 | 31.3±12.8 | 34.9±13.5 | 0.350 |
| Post-PMV | 24.7±8.5 | 23.2±7.6 | 30.0±10.8 | 0.010 |
| PCW pressure | ||||
| Pre-PMV | 23.7±7.8 | 23.1±7.7 | 26.5±8.1 | 0.140 |
| Post-PMV | 18.3±7.3 | 17.3±6.6 | 20.8±8.9 | 0.130 |
| LA pressure | ||||
| Pre-PMV | 22.8±7.6 | 22.6±7.5 | 24.3±7.4 | 0.450 |
| Post-PMV | 16.9±7.5 | 16.5±6.3 | 19.6±11.0 | 0.170 |
| End-diastolic LV pressure | ||||
| Pre-PMV | 13.5±9.3 | 13.4±10.5 | 14.1±5.0 | 0.800 |
| Post-PMV | 14.6±6.7 | 14.0±6.8 | 16.3±6.2 | 0.240 |
| Systolic LV pressure | ||||
| Pre-PMV | 133.6±26.8 | 134.5±27.4 | 132.4±27.7 | 0.790 |
| Post-PMV | 128.3±28.4 | 127.7±29.4 | 134.1±26.8 | 0.440 |
CO: cardiac output; LA: left atrial; LV: left ventricular; MVA: mitral valve area; MG: mitral gradient; MR: mitral regurgitation; PA: pulmonary artery; PCW: pulmonary capillary wedge pressure; PMV: percutaneous mitral valvuloplasty; RA: right atrial; RV: right ventricular. All pressures and gradients are in mmHg.
Procedural success was achieved in 75 of 91 (82.4%) patients, using the above-mentioned definition. The 16 inadequate immediate results were related to suboptimal valve opening (valve area <1.5cm2) in 12 cases (75%) and severe MR (grade≥3) in four cases (25%).
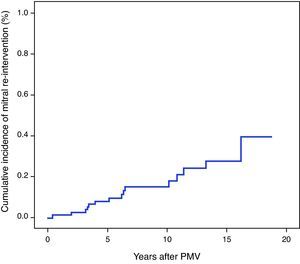
Mean pre-PMV MVA and MG were 1.0±0.3cm2 and 12.3±5.2mmHg and improved to 2.3±0.4cm2 and 3.0±3.8mmHg after PMV, respectively (p<0.001 for both). The median time to mitral re-intervention was 6.2 (3.3–10.8) years.
The 1-, 3-, 5-, 7- and 9-year rates of post-PMV mitral re-intervention-free survival were 98.8±1.2%, 97.5±1.7%, 92.1±3.1%, 85.5±4.5%, and 85.5±4.5%, respectively (Figure 1). Regarding the second endpoint, the 1-, 3-, 5-, 7- and 9-year rates of clinical event-free survival were 93.0±2.8%, 86.0±3.9%, 81.0±4.4%, 70.6±5.6%, and 68.4±5.8%, respectively.
During follow-up, nine patients died, four due to cardiovascular causes. Fifteen patients underwent mitral valve open heart surgery (eight underwent mitral valve replacement with a mechanic prosthesis and seven mitral valve repair) and four underwent repeat PMV; one patient underwent both procedures. In addition, eight hospital admissions due to decompensated heart failure were recorded. No deaths occurred in the group that underwent post-PMV mitral re-intervention.
We therefore analyzed the group of patients with long-term follow-up available who had not proceeded immediately to surgery due to severe iatrogenic mitral regurgitation (n=82).
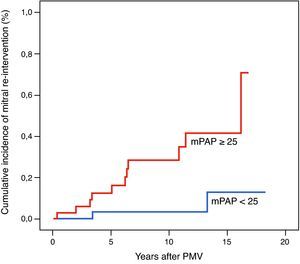
Regarding clinical characteristics, patients who required mitral re-intervention during follow-up were younger (43.3±12.8 vs. 51.2±13.8 years, p=0.04) and had higher pre-PMV MG (14.9±5.1 vs. 11.5±5.0mmHg, p=0.02), post-PMV MG (6.4±6.8 vs. 2.1±2.2mmHg, p<0.001) and post-PMV mean pulmonary artery pressure (mPAP) (30.0±10.8 vs. 23.2±7.6mmHg, p=0.01). Using a Cox proportional hazards model, mPAP was the only predictor of post-PMV mitral re-intervention (hazard ratio [HR] 5.639 [95% CI 1.246–25.528], p=0.025) (Table 3). The best immediate post-PMV mPAP cut-off value for predicting mitral re-intervention was 25mmHg, with a sensitivity of 81% (95% CI 41–89) and a specificity of 57% (95% CI 49–75). Patients with immediate post-PMV mPAP<25mmHg showed significantly higher mitral re-intervention-free survival rates than those with post-PMV mPAP≥25mmHg (94.1% vs. 68.6%), log-rank p=0.01 (Figure 2). Mean PAP≥25mmHg also had independent predictive value for the occurrence of the composite endpoint (HR 3.622 [1.070–12.260], p=0.039) (Table 3). Patients with post-PMV mPAP<25mmHg had significantly higher clinical event-free survival rates than those with post-PMV mPAP≥25mmHg (88.2% vs. 60.0%), log-rank p=0.007.
Results of Cox proportional hazards multivariable analysis.
| Variable | Post-PMV mitral re-intervention (endpoint 1) | Clinical events composite endpoint (endpoint 2) | ||
| HR (95% CI) | p | HR (95% CI) | p | |
| Age (years) | 0.984 (0.933–1.037) | 0.541 | 0.998 (0.961–1.036) | 0.907 |
| Body mass index (kg/m2) | 0.836 (0.683–1.023) | 0.082 | 0.996 (0.864–1.148) | 0.955 |
| Pre-PMV MG ≥13mmHg | 0.994 (0.212–4.665) | 0.994 | 2.648 (0.913–7.681) | 0.073 |
| Post-PMV mPAP ≥25mmHg | 8.259 (1.378–49.941) | 0.021 | 3.622 (1.070–12.260) | 0.039 |
| Post-PMV MG ≥2.5mmHg | 3.123 (0.721–13.519) | 0.128 | 0.935 (0.313–2.792) | 0.904 |
CI: confidence interval; HR: hazard ratio; MG: mitral gradient; mPAP: mean pulmonary artery pressure; PMV: percutaneous mitral valvuloplasty.
PMV is an effective and safe method of relieving MS. However, sooner or later, a proportion of patients require re-intervention due to restenosis of the mitral orifice or increasing severity of MR. We analyzed this group of patients and performed a comparison with patients who did not require such intervention and used a clinical endpoint chosen for its relevance to patients, as opposed to a purely echocardiographic parameter such as restenosis, which can occur in relatively asymptomatic patients and consequently has little clinical value.
We defined successful PMV as MVA≥1.5cm2 and MR≤2/4. Since MS patients with MVA≥1.5cm2 are generally considered to have mild stenosis and are relatively asymptomatic, this value was chosen as the threshold for procedural success.16 Using this definition, we observed a PMV procedural success rate of 82.4%. Other studies, using the same definition, reported success rates ranging from 70 to 90%.3,17 In all patients, PMV was performed with the Inoue balloon technique. The main advantages of this technique (versus the double-balloon technique) are the absence of a guidewire in the left ventricle, the self-positioning of the balloon, and the ability to perform stepwise inflations under echo guidance to monitor commissural opening.18 MR remains one of the most important complications of this procedure. In our population, 4.5% of patients (n=4) developed significant MR after PMV, with three requiring mitral surgery. Differences in patient populations, selection criteria for significant MR, and PMV technique (double-balloon vs. Inoue balloon technique) may explain the wide range of significant MR rates reported in the literature (7.5–15.0%).19–22
It is generally accepted that patients with echo scores <8 have a better outcome with PMV.3 Our study reports a median score of 6 (5–7), with eight patients presenting with a score of 8. Previous studies identified echo score as an independent predictor of surgery.23 However, this was not the case in our population as no differences were found between the groups; this observation is probably related to the fact that all patients included had an echo score of ≤8.
In our series mitral re-intervention-free survival rates were similar to other published reports, even considering the fact that in some patients (17.6%) the procedure was not successful according to the above definition.12 Regarding clinical event-free survival, we decided to include decompensated heart failure in our composite clinical endpoint, in addition to mitral re-intervention and cardiovascular death, as this is a significant cardiac event which causes significant burden in terms of quality of life and use of health resources. Probably due to the fact that most series do not include decompensated heart failure as an endpoint, our event-free rates are slightly lower than those reported by other groups.12
Within the group that required post-PMV mitral re-intervention, four patients repeated PMV, eight underwent mitral valve replacement and seven mitral repair. All those undergoing mitral valve replacement had a mechanical device implanted. This is probably related to two factors. First, given the relatively young age of our study group, implanting a mechanical valve could avoid future operations. Second, a significant proportion of these patients were in atrial fibrillation and on warfarin, so a decision for life-long anticoagulation due to a mechanical valve would have been easier to take. No deaths were observed in the group undergoing mitral re-intervention.
The present study found that immediate post-PMV mPAP was the only independent predictor of the two predefined endpoints, with the best cut-off value for predicting mitral re-intervention being 25mmHg. The most commonly reported independent predictors of post-PMV clinical events (cardiovascular death, mitral valve surgery and repeat PMV) and restenosis are echo score and post-PMV MVA, both related to mitral valve anatomy.2,6,7,12,23 However, our results are in agreement with similar studies that also identified mPAP as an independent predictor of event-free survival.2,6 Differences in the definition of endpoints and in baseline population characteristics may explain the different predictors reported in the literature.
In mitral stenosis, pulmonary hypertension (PH) is due to three components: (1) passive, due to left atrial hypertension; (2) vasoreactive, due to pulmonary arteriolar constriction; and (3) due to structural changes in pulmonary vascular disease.24–26 The first two are reversible, the first immediately after PMV and the second later, within a few months of intervention.27 The third factor is permanent and does not usually regress.28
In our population, no differences were found in pre- and post-PMV MVA between the two groups of patients. This lack of association between relief of mitral obstruction and improvement in pulmonary hemodynamics has been seen in other studies.25,29 Patients who underwent mitral re-intervention had higher post-PMV mPAP. The initial decrease in mPAP observed post-PMV is probably due to an immediate decrease in left atrial pressure and the remainder is due to the second and third components described above. Indeed, and as reported in other studies, the gain in MVA is not predictive of a decrease in PA pressure. Despite similar MVA and echo scores, patients who required mitral re-intervention during follow-up had higher mPAP. These findings may suggest that mPAP may be an important marker of disease severity, probably reflecting the chronicity of the disease, as the duration of stenosis is an important factor in the development of reactive PH.30 Although the vasoreactive component of PH in mitral stenosis may improve in the first few months after the procedure and consequently will have no influence on long-term outcome following PMV, the fact remains that patients with higher mPAP immediately after the procedure had a higher incidence of post-PMV re-intervention. This is probably due to a predominant component of non-reversible vascular remodeling in these patients, reflecting longer and/or more severe mitral flow restriction. Closer post-PMV follow-up should be considered in these patients.
Although our study represents the largest cohort of PMV patients with long-term follow-up data reported to date in Portugal, it has several shortcomings. First, the study reflects a single-center experience and includes a relatively small number of patients, all with an echo score under 8, reflecting the selected nature of the population. Second, regular long-term echocardiographic follow-up was not possible in a significant proportion of patients, so no data regarding long-term mPAP were available. We used Seller's qualitative criteria to assess MR, rather than more accurate quantitative echocardiographic indices, and were thus unable to account for the post-procedural MR mechanism. However, we believe that this is not likely to affect our main results and conclusions.
ConclusionsIn conclusion, in our population, immediate post-PMV mPAP was the sole predictor of post-PMV mitral re-intervention. The best immediate mPAP cut-off value for predicting mitral re-intervention was 25mmHg. These findings may help identify patients with more severe disease with a higher probability of requiring mitral re-intervention. Closer post-PMV follow-up should be considered in these high-risk patients.
Conflicts of interestThe authors have no conflicts of interest to declare.