Risk stratification of Brugada syndrome (BrS) remains controversial and recommendations for an implantable cardioverter-defibrillator (ICD) are not well established. The objective of this study was to assess the long-term prognosis of BrS patients with an ICD.
Methods and ResultsOf 55 consecutive patients with BrS assessed between April 2002 and October 2012, 36 (mean age 41.7±13.8 years; 81.8% male) underwent ICD implantation. Nineteen (52.8%) were asymptomatic, 11 (30.6%) had previous history of syncope (arrhythmic cause suspected in eight) and six (16.7%) had aborted sudden cardiac death (SCD). Spontaneous type 1 electrocardiographic (ECG) pattern was present in 25 (69.4%) patients and electrophysiological study (EPS), performed in 26 (72.2%), was positive in 22 (84.6%). During a mean follow-up of 74±40 months (>5 years in 72% of cases), seven (19.4%) patients had appropriate shocks (annual event rate 2.8%). These patients most frequently had aborted SCD (54.1% vs. 6.9%; p=0.008) and nonsustained ventricular tachycardia (57.1% vs. 10.3%; p=0.016) during follow-up. Spontaneous type 1 ECG pattern, syncope and positive EPS were not significantly associated with appropriate shocks. Multivariate analysis revealed that aborted SCD was an independent predictor of appropriate shocks (HR 8.07, 95% CI 1.58–41.2; p=0.012). ROC curve analysis demonstrated that aborted SCD had moderate discriminatory power to predict appropriate shocks (AUC 0.751), with sensitivity of 57% and specificity of 93%. In terms of ICD-related complications, eight (22.2%) patients had inappropriate shocks during the follow-up period, mainly due to sinus tachycardia (five patients); one patient had lead infection and another had a lead fracture.
ConclusionIn this population of BrS patients with ICD, the long-term rate of appropriate shocks was 2.8%/year. Aborted SCD was associated with a higher risk of appropriate shocks, whereas syncope and spontaneous type I ECG pattern did not predict this event.
A estratificação de risco na síndrome de Brugada (SB) permanece controversa e as recomendações para cardiodesfibrilhador (CDI) não estão bem definidas. O objetivo deste estudo foi avaliar o prognóstico a longo prazo de doentes com SB e CDI implantado.
Métodos e resultadosDe 55 doentes consecutivos com SB avaliados entre abril/2002-outubro/2012, 36 (idade média 41,7±13,8 anos; 81,8% homens) implantaram CDI. Dezanove (52,8%) eram assintomáticos, 11 (30,6%) tinham história de síncope (oito com suspeita de causa arrítmica) e seis (16,7%) foram reanimados de morte súbita (MS). O eletrocardiograma (ECG) com padrão tipo 1 espontâneo estava presente em 25 (69,4%) doentes e foi realizado estudo eletrofisiológico (EEF) em 26 (72,2%), sendo positivo em 22 (84,6%). Durante o período de seguimento médio de 74±40 meses (>5 anos em 72% dos casos), sete (19,4%) doentes tiveram choques apropriados (incidência anual 2,8%). Estes doentes tinham mais frequentemente história de MS abortada (54,1 versus 6,9%; p=0,008) e taquicardia não mantida (57,1 versus 10,3%; p=0,016) durante o seguimento. Padrão ECG tipo 1 espontâneo, síncope e EEF positivo não se associaram significativamente à ocorrência de choques apropriados. Em análise multivariada a MS abortada manteve-se preditor independente de choques apropriados (HR 8,07 IC95% 1,58-41,2; p=0,012). Em análise de curvas ROC a MS abortada apresentou um poder discriminatório moderado para predizer choques apropriados (AUC 0,751) – sensibilidade 57% e especificidade 93%. Relativamente às complicações relacionadas com o CDI, oito (22,2%) doentes tiveram choques inapropriados durante o período de seguimento, sobretudo por taquicardia sinusal (cinco doentes), um infeção de elétrodo e outro fratura de elétrodo.
ConclusãoNa população estudada de doentes com SB e CDI implantado a incidência de choques apropriados foi 2,8%/ano. A MS abortada associou-se a um maior risco de choques apropriados, enquanto a síncope e o padrão de ECG tipo 1 espontâneo não foram preditores deste evento.
Brugada syndrome is an inherited arrhythmogenic heart disease characterized by an increased risk of sudden cardiac death (SCD) due to ventricular tachycardia (VT)/ventricular fibrillation (VF). The syndrome accounts for approximately 4% of all SCD and up to 20% of sudden deaths in patients with structurally normal hearts.1 Several series have demonstrated a significant recurrence rate of malignant arrhythmic events in high-risk patients, suggesting that implantable cardioverter-defibrillator (ICD) implantation is beneficial.2–4 Risk stratification is thus a central step in assessment of patients with Brugada syndrome, allowing for the identification of individuals at higher risk of arrhythmic events who would benefit from an ICD. The problem is that there is still controversy as to how to stratify these patients and doubts as to which patients should receive a prophylactic ICD.
The most common variables associated with a higher risk of arrhythmic events in Brugada syndrome patients are aborted SCD, history of syncope and spontaneous type 1 electrocardiographic (ECG) pattern, but the role of the electrophysiological study (EPS) is not well established due to contradictory results in the published literature.4,5
There is consensus for the implantation of an ICD in cardiac arrest survivors, and there is general agreement that patients with a spontaneous ECG pattern and a history of syncope or documented VT that has not resulted in SCD should receive an ICD.6–8 At the other end of the spectrum, in low-risk patients (asymptomatic with type 1 ECG only after pharmacological provocation), clinical follow-up is also generally accepted.6–8 However, in patients with syncope and pharmacologically induced type 1 ECG or asymptomatic patients with spontaneous type 1 ECG, whether to implant a prophylactic ICD is currently a major dilemma.
In this study, the main objectives were to assess the long-term prognosis of Brugada syndrome patients with an ICD implanted for primary or secondary prevention of SCD and to identify independent predictors of appropriate shocks during follow-up.
MethodsPopulationFifty-five consecutive patients with Brugada syndrome were assessed retrospectively in a single tertiary center between April 2002 and October 2012. A diagnosis of Brugada syndrome was established after an episode of aborted SCD, during investigation of syncope, in asymptomatic patients during routine clinical examination with typical ECG patterns, or during family screening.
Diagnosis, clinical assessment and electrophysiological studyThe following clinical data of interest were recorded: demographic characteristics (gender, age and race); clinical presentation (aborted SCD and syncope); and family history of SCD and nonsustained ventricular tachycardia (NSVT) during follow-up. ECGs were reviewed and classified by two physicians according to the consensus report of current electrocardiographic criteria for diagnosis of Brugada pattern.9 Routine examinations included transthoracic echocardiogram for exclusion of structural cardiac disease and laboratory tests for exclusion of metabolic and electrolyte abnormalities. Intravenous flecainide, a sodium-channel-blocking agent, was used for provocative pharmacological tests (2 mg/kg bodyweight over 10 min with a maximum dose of 150 mg). EPS was performed with two drive cycles (600 and 400 ms, S1) and a maximum of three ventricular extrastimuli (S2, S3 and S4) delivered from two different ventricular sites (right ventricular apex and right ventricular outflow tract), unless VF or sustained VT (lasting >30 s), causing syncope, or requiring intervention for termination, was elicited in a previous step. Minimum coupling interval was 200 ms for S2 to S4. A positive EPS was defined as induced VF, sustained polymorphic VT or polymorphic syncopal VT requiring direct current shock.
Implantable cardioverter-defibrillator implantation and follow-upICDs were implanted according to the criteria recommended in the second consensus report on Brugada syndrome.6 Implantation was performed with a transvenous system in all patients, through the cephalic or left subclavian vein at the operator's discretion. It is our routine protocol to program a single VF zone with a detection rate of 180–200 bpm and backup pacing at a rate of 45 bpm for these patients. Appropriate shocks were defined as shocks delivered for VT or VF; inappropriate shocks were defined as shocks delivered in the absence of ventricular arrhythmia. The main endpoint assessed during a mean follow-up of 74±40 months (>5 years in 72% of cases) was the occurrence of appropriate shocks.
Statistical analysisContinuous variables were expressed as mean ± standard deviation. Discrete variables were expressed as frequencies and percentages. Statistical comparisons of baseline characteristics and outcomes were performed using the chi-square test or Fisher's exact test, as appropriate, for categorical variables and the Student's t test for continuous variables. Corrected risk estimates were performed using a Cox proportional hazard regression model including the following variables: spontaneous type 1 ECG pattern, history of syncope, aborted SCD and NSVT during follow-up. For this analysis the time to first appropriate shock was considered. Receiver operating characteristic (ROC) analysis was performed with determination of the area under the curve (AUC) and respective specificity, sensitivity, positive and negative predictive values. Two-tailed tests of significance are reported. For all comparisons, a p value of <0.05 was considered statistically significant. When appropriate, 95% confidence intervals (CI) were calculated. The statistical analysis was performed with SPSS version 19.0 (SPSS Inc., Chicago, IL, USA).
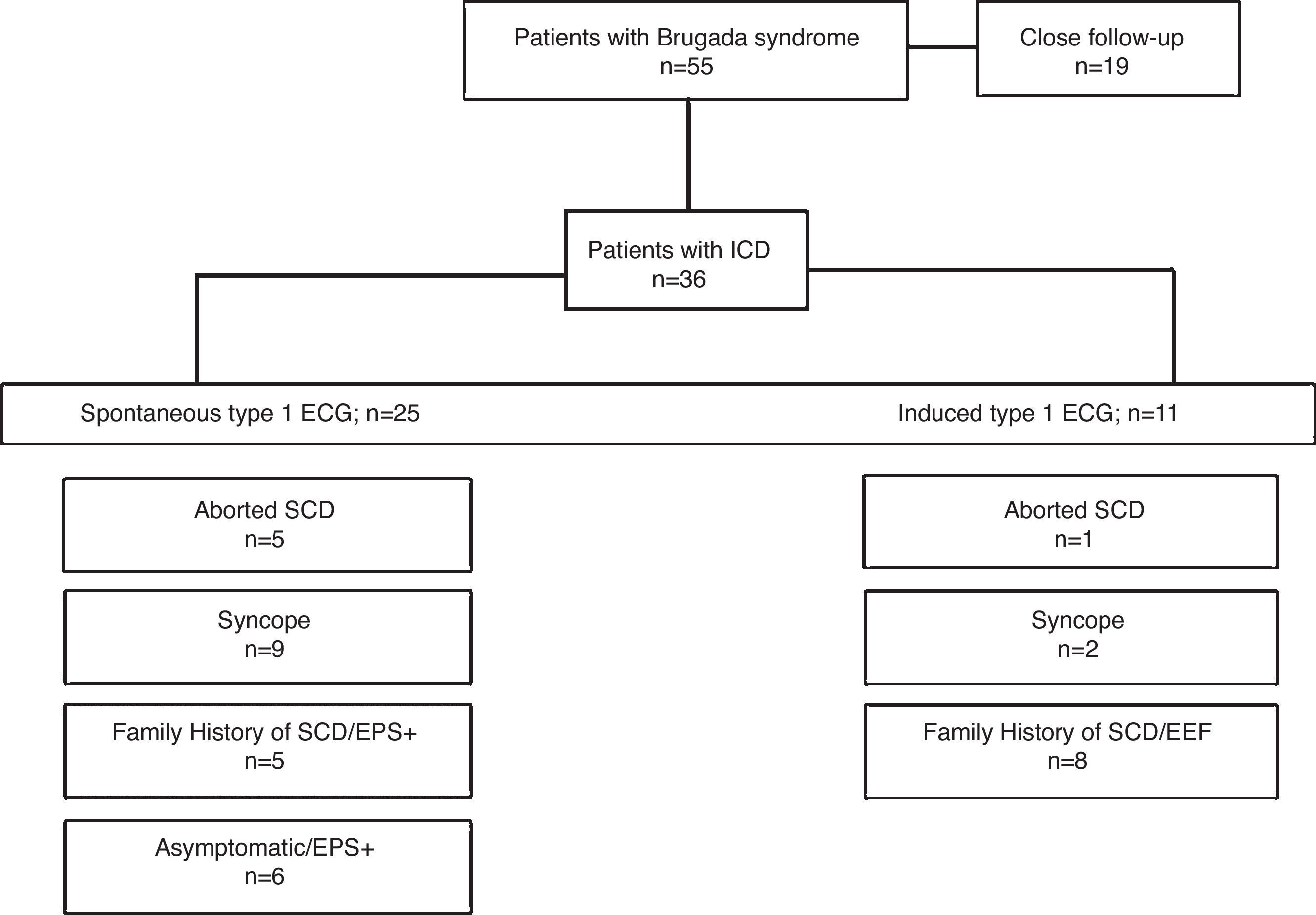
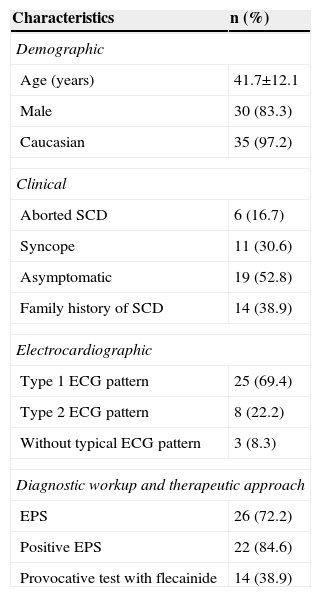
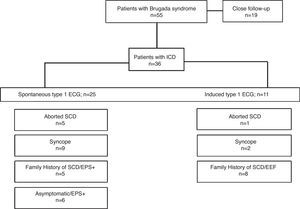
ResultsPatient characteristicsOf the 55 patients with Brugada syndrome included in the present analysis, 36 (65.5%; mean age 41.7±13.8 years; 81.8% male) underwent ICD implantation. The baseline characteristics of these patients are depicted in Table 1. Briefly, 19 (52.8%) patients were asymptomatic, 11 (30.6%) had a previous history of syncope (with a possible arrhythmic cause suspected in eight patients), six (16.7%) had aborted SCD and 14 (38.9%) had a family history of SCD. Spontaneous type 1 ECG pattern was present in 25 (69.4%) patients. EPS was performed in 26 (72.2%) patients, with positive results in 22 (84.6%). Provocative testing with flecainide was performed in 14 (38.9%) patients. Indications for ICD implantation are represented in Figure 1.
Baseline characteristics of patients with ICD (n=36).
| Characteristics | n (%) |
|---|---|
| Demographic | |
| Age (years) | 41.7±12.1 |
| Male | 30 (83.3) |
| Caucasian | 35 (97.2) |
| Clinical | |
| Aborted SCD | 6 (16.7) |
| Syncope | 11 (30.6) |
| Asymptomatic | 19 (52.8) |
| Family history of SCD | 14 (38.9) |
| Electrocardiographic | |
| Type 1 ECG pattern | 25 (69.4) |
| Type 2 ECG pattern | 8 (22.2) |
| Without typical ECG pattern | 3 (8.3) |
| Diagnostic workup and therapeutic approach | |
| EPS | 26 (72.2) |
| Positive EPS | 22 (84.6) |
| Provocative test with flecainide | 14 (38.9) |
ECG: electrocardiogram; EPS: electrophysiological study; SCD: sudden cardiac death.
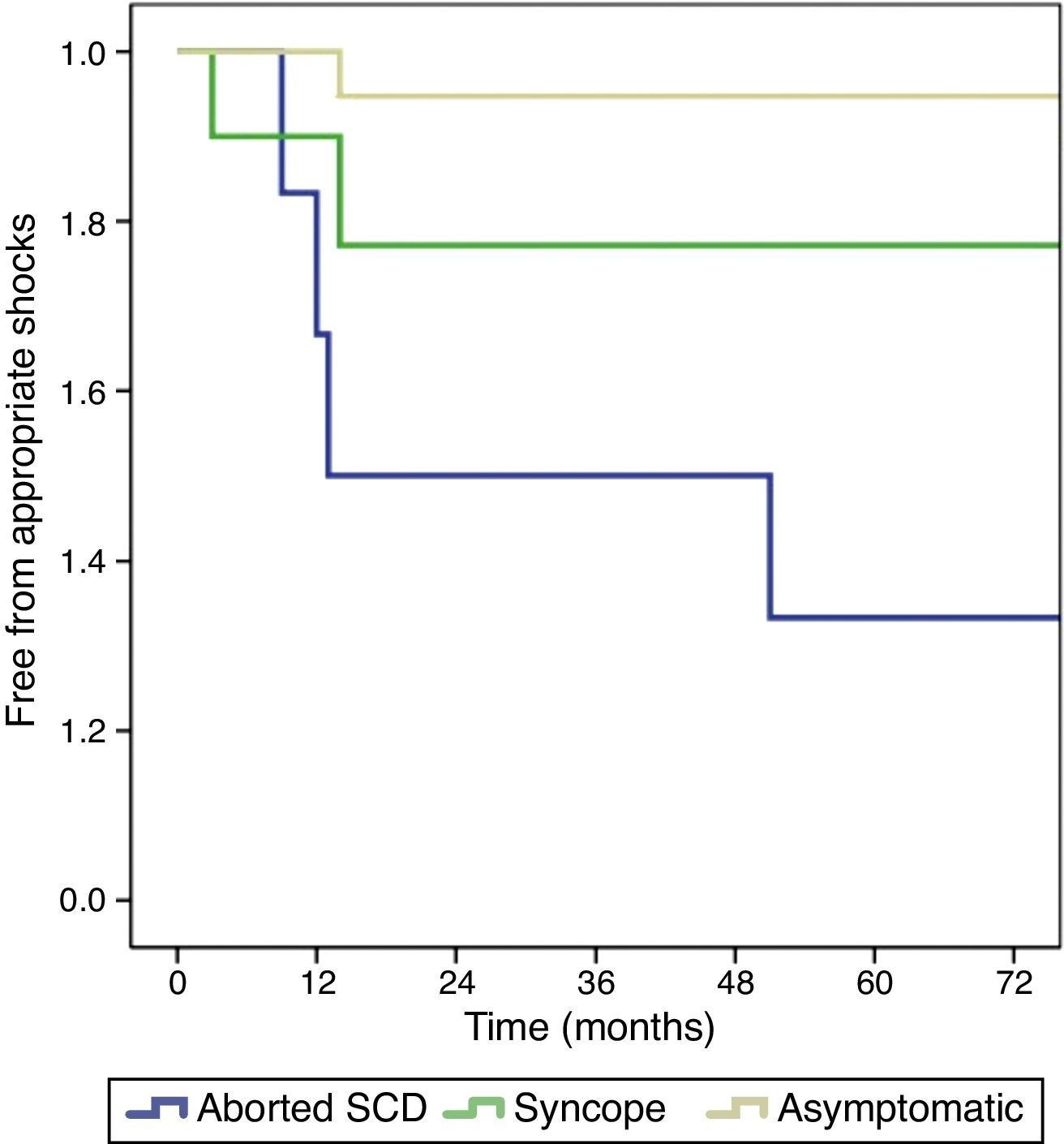
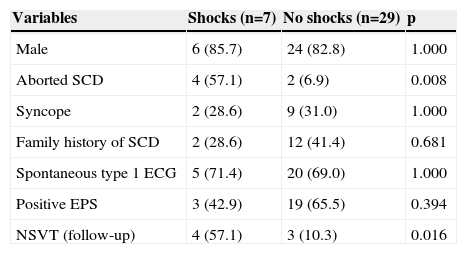
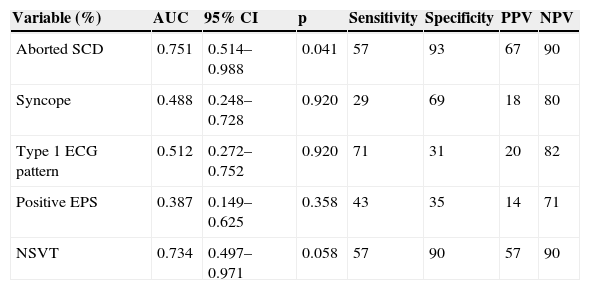
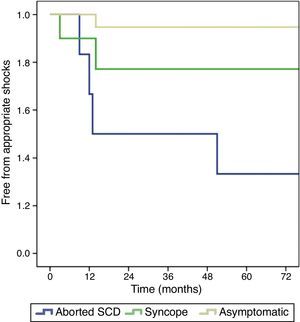
During a mean follow-up of 74±40 months (>5 years in 72% of cases), seven patients experienced appropriate shocks, corresponding to an incidence of 19.4% and an annual event rate of 2.8%. Four patients had arrhythmic storm (successfully treated with quinidine). Median time to the first appropriate shock was 17 months, all events occurring between 3 and 51 months after ICD implantation. With regard to clinical presentation, the rate of appropriate shocks was significantly lower in asymptomatic patients than in those with a history of syncope and aborted SCD: 10.5%, 18.2% and 66.7%, respectively (p=0.007) (Figure 2). The majority of patients with appropriate shocks were men, with aborted SCD and spontaneous type 1 ECG pattern (Figure 3). Comparing patients with and without appropriate shocks, the first group had a statistically significant higher prevalence of aborted SCD (54.1% vs. 6.9%, p=0.008) and NSVT (57.1% vs. 10.3%, p=0.016) during follow-up. Spontaneous type 1 ECG pattern, family history of SCD, syncope and positive EPS did not present a statistically significant association with appropriate shocks (Table 2). In the two patients with syncope at presentation and appropriate shocks during follow-up, an arrhythmic cause for syncope was suspected. Multivariate analysis adjusted for statistically significant characteristics in univariate analysis (aborted SCD and NSVT during follow-up) and for spontaneous type 1 ECG pattern and history of syncope revealed independent predictors of appropriate shocks to be aborted SCD (hazard ratio [HR] 7.87, 95% CI 1.27–49.6; p=0.027) and NSVT during follow-up (HR 6.73, 95% CI 1.27–35.7; p=0.025). By ROC curve analysis, aborted SCD had moderate discriminatory power to predict the occurrence of appropriate shocks (AUC=0.751), with sensitivity and specificity of 57% and 93%, respectively (Table 3). None of the patients died during follow-up. In terms of ICD-related complications, eight (22.2%) patients had inappropriate shocks during the follow-up period, mainly due to sinus tachycardia (five patients), mainly managed by increasing ICD thresholds or by beta-blocker therapy. One patient had documented lead infection and underwent device replacement and another had a lead fracture.
Comparison of patients according to the occurrence of appropriate shocks.
| Variables | Shocks (n=7) | No shocks (n=29) | p |
|---|---|---|---|
| Male | 6 (85.7) | 24 (82.8) | 1.000 |
| Aborted SCD | 4 (57.1) | 2 (6.9) | 0.008 |
| Syncope | 2 (28.6) | 9 (31.0) | 1.000 |
| Family history of SCD | 2 (28.6) | 12 (41.4) | 0.681 |
| Spontaneous type 1 ECG | 5 (71.4) | 20 (69.0) | 1.000 |
| Positive EPS | 3 (42.9) | 19 (65.5) | 0.394 |
| NSVT (follow-up) | 4 (57.1) | 3 (10.3) | 0.016 |
ECG: electrocardiogram; EPS: electrophysiological study; NSVT: non-sustained ventricular tachycardia; SCD: sudden cardiac death.
Predictive value for appropriate shocks of different characteristics.
| Variable (%) | AUC | 95% CI | p | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|
| Aborted SCD | 0.751 | 0.514–0.988 | 0.041 | 57 | 93 | 67 | 90 |
| Syncope | 0.488 | 0.248–0.728 | 0.920 | 29 | 69 | 18 | 80 |
| Type 1 ECG pattern | 0.512 | 0.272–0.752 | 0.920 | 71 | 31 | 20 | 82 |
| Positive EPS | 0.387 | 0.149–0.625 | 0.358 | 43 | 35 | 14 | 71 |
| NSVT | 0.734 | 0.497–0.971 | 0.058 | 57 | 90 | 57 | 90 |
AUC: area under the curve; ECG: electrocardiogram; EPS: electrophysiological study; PPV: positive predictive value; NPV: negative predictive value; NSVT: non-sustained ventricular tachycardia; SCD: sudden cardiac death.
During the mean follow-up of 69±31 months, the 19 patients without ICD implantation were alive, and only one underwent ICD implantation, without occurrence of appropriate shocks.
DiscussionBrugada syndrome is an arrhythmogenic disease associated with a high risk of SCD in young individuals with structurally normal hearts, and is classically characterized by an ECG phenotype of right bundle branch block and ST-segment elevation in the right precordial leads.1,9 The prevalence of the syndrome is difficult to estimate since ECG findings can be dynamic and/or concealed, and there are potentially endemic regions for the genetic alterations associated with this entity (mutations in the SCN5A sodium channel gene). Its prevalence is estimated at approximately 5/10 000 individuals, with a higher prevalence in Asia.10–13
Risk stratification of SCD is essential to the management of patients with Brugada syndrome. The objective is to identify patients at higher risk of SCD who may benefit from ICD implantation, the only measure with proven survival benefit in these patients.8 A substantial part of the evidence for the advantages of an ICD comes from the results of the DEBUT trial.14 No pharmacological therapy has shown to improve survival in Brugada syndrome patients, but class IA antiarrhythmic drugs, particularly quinidine, which inhibit the potassium transient outward current of the action potential, may be useful in refractory cases.15
In the present study, performed in patients with Brugada syndrome who received an ICD for primary or secondary prevention, the long-term (mean follow-up of 6.2 years) annual event rate of potentially life-threatening ventricular arrhythmias was 2.8%/year. The rate of appropriate ICD shocks was similar to results previously published by Rosso et al.16 and Sacher et al.,17 despite the longer follow-up in our analysis.
Several characteristics have been described as predictors of the occurrence of arrhythmic events in Brugada syndrome patients. Aborted SCD, history of syncope, spontaneous type 1 ECG pattern and positive EPS are associated with increased risk.2,18 However, distribution of these characteristics among different published studies is not homogeneous. In the FINGER registry,19 during a mean follow-up of 32 months, cardiac events (appropriate shocks or SCD) were recorded in 5% of Brugada patients. Independent predictors of the event were aborted SCD, syncope and spontaneous type 1 ECG. In fact, a history of syncope and aborted SCD are the most common clinical manifestations in Brugada syndrome patients, predominantly manifested in men during the third and fourth decades of life.15 Nevertheless, a significant number of patients with Brugada syndrome are asymptomatic at the time of diagnosis. These patients have a more favorable long-term prognosis compared with those with symptomatic presentation (e.g. aborted SCD, syncope or symptomatic VT).20
When analyzed in a multivariate model, aborted SCD and NSVT during follow-up were the only independent predictors of appropriate shocks in our analysis. Aborted SCD had moderate discriminatory power to predict this outcome. It is important to recall that an appropriate shock is not synonymous with SCD. Although a proven relation between aborted SCD and the risk of arrhythmic events in Brugada syndrome patients has been frequently reported, an independent association of NSVT with appropriate shocks during follow-up has also been described.21 However, it should be borne in mind that NSVT episodes saved in the ICD data log are dependent on the heart rate and duration in the device programming.
The association between syncope and the occurrence of appropriate shocks remains somewhat controversial.22,23 In our study, syncope was the major symptom at diagnosis, mainly of arrhythmic origin, but was not significantly associated with the occurrence of appropriate shocks at long-term follow-up. Similarly to our results, in a study by Sarkozy et al.,24 syncope was not a predictor of arrhythmic events after prophylactic ICD implantation. Unfortunately, sometimes it is not easy to differentiate the origin of syncope between benign (reflex) or potentially fatal (VT). This difficulty suggests that patients with benign syncope may have comprised a significant part of our syncopal population, which may have distorted the results. In another analysis of 203 patients with Brugada syndrome,25 the prevalence of syncope was 28% and in many cases a cardiac etiology was considered unlikely. The follow-up of these patients showed that long-term arrhythmic events occurred only in patients with syncope of cardiac origin. Similarly, in our study events occurred only in patients with possible arrhythmic syncope.
Type 1 ECG pattern and inducibility during EPS were not predictors of appropriate shocks. The role of EPS in risk stratification and management of Brugada syndrome patients is not well established, with published data revealing conflicting results. Brugada et al.5 suggest that VT/VF induction in asymptomatic patients is significantly correlated with an increased risk of SCD; on the other hand, Priori et al.4 suggest that EPS does not add significant value to risk stratification in either symptomatic or asymptomatic patients. These discrepancies may be explained by the differences in baseline characteristics of the study populations and the different stimulation protocols. In fact, it is known that programmed stimulation induces VF in 6–9% of apparently healthy individuals, representing false positives, particularly when more aggressive protocols are used.26,27 Paul et al.28 performed a meta-analysis to assess the role of programmed ventricular stimulation in patients with Brugada syndrome, and showed that EPS did not have a significant role in predicting arrhythmic events during follow-up.
In our study a family history of SCD in first-degree relatives was not associated with an increased risk of arrhythmic events during follow-up. This finding is in agreement with the results of several previously published studies.17,19,24 A positive provocative pharmacological test with flecainide also failed to identify patients with increased risk of appropriate shocks, as described in the PRELUDE Registry.18
Our results support both the importance of ICD implantation in SCD survivors and the current non-invasive strategy of close follow-up, without device implantation, in asymptomatic patients. In patients with syncope, discrepancies from previous results highlight the need for better discrimination of the nature of syncope (arrhythmic vs. reflex).
Only two patients had severe complications associated with ICD implantation (infection and lead fracture) requiring device replacement, while eight patients had inappropriate shocks during follow-up. Given the young age and active lifestyle of our patients, many engaging in regular sports, it is not surprising that the leading cause of inappropriate shocks was sinus tachycardia.17,19 The rate of ICD complications exceeding the rate of arrhythmic events during follow-up has been previously reported,21,29 highlighting the importance of an accurate risk stratification scheme. Inappropriate patient selection for ICD implantation may result in a considerable increase in healthcare costs and may expose asymptomatic individuals to ICD-related complications.17
The present study has some limitations. It is retrospective and the small number of patients and the low event rate during follow-up make it difficult to draw any definitive conclusions regarding predictors of long-term outcome. Some ventricular tachyarrhythmias may terminate spontaneously, depending on how the device is programmed.
ConclusionsLong-term prognostic analysis of patients with Brugada syndrome and an ICD implanted for primary or secondary prevention revealed an annual event rate of potentially life-threatening ventricular arrhythmias of 2.8%/year. Aborted SCD and NSVT during follow-up were independent predictors of the occurrence of appropriate shocks, whereas syncope, type 1 ECG pattern, EPS and family history of SCD were not associated with a higher rate of events in this population. The number of patients with inappropriate shocks was similar to the number of patients with appropriate shocks, and two patients had serious ICD-related complications. Risk stratification of patients with Brugada syndrome remains controversial and the risk/benefit ratio of this therapy should be taken into account.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.