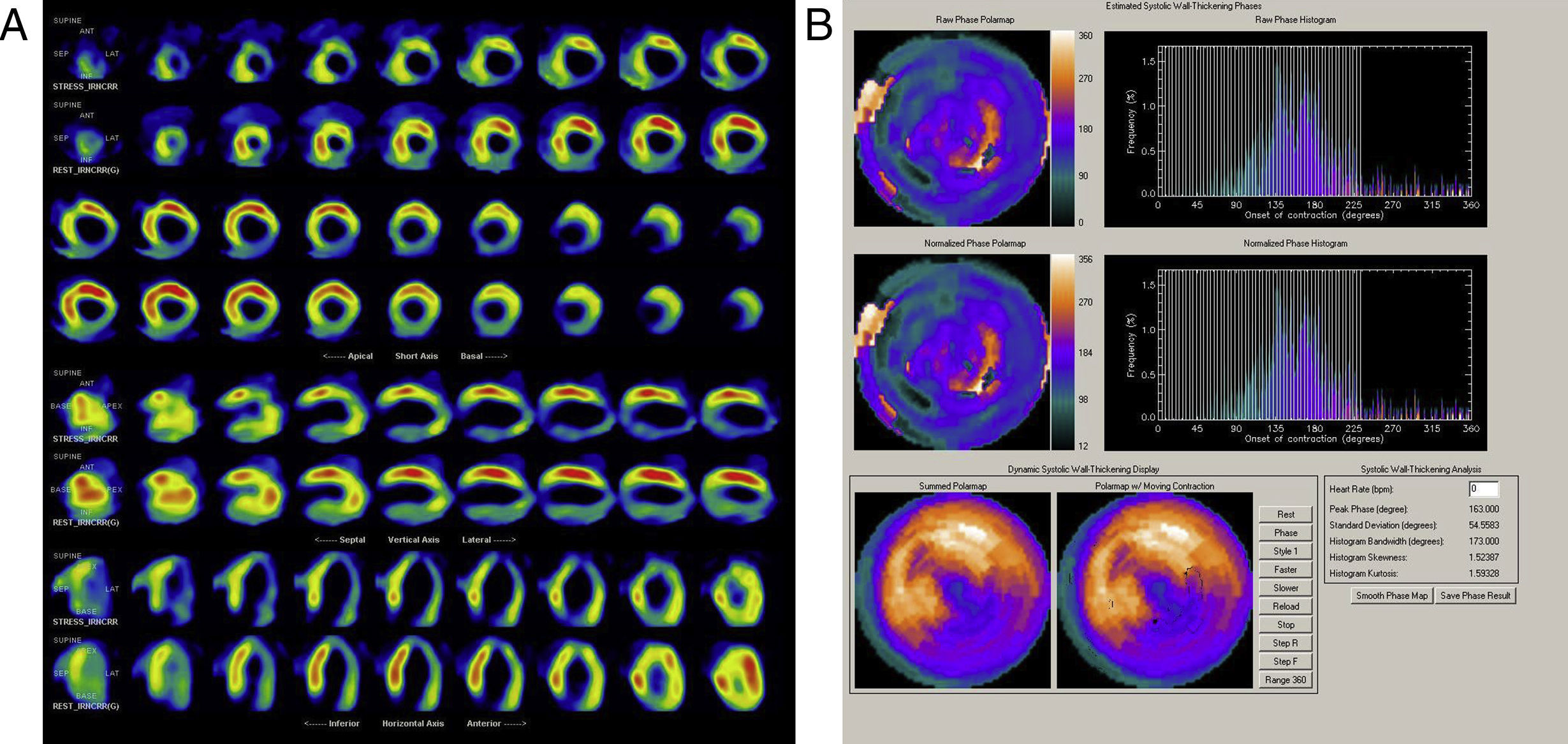
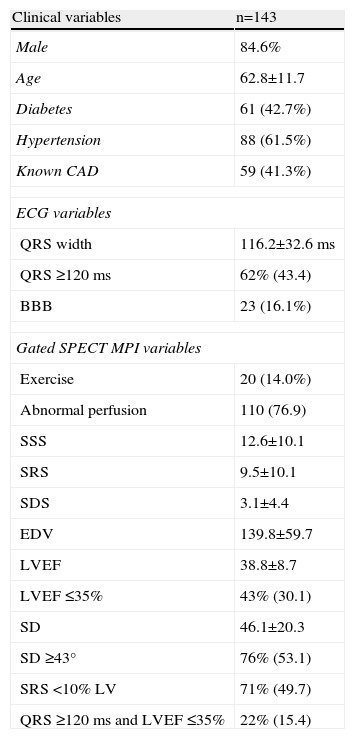
Gated SPECT myocardial perfusion imaging (MPI) has been used to quantify mechanical dyssynchrony. Mechanical dyssynchrony appears to be related to response to cardiac resynchronization therapy.
ObjectiveTo evaluate the presence and predictors of mechanical dyssynchrony in patients with impaired left ventricular function (LVEF) ≤50%.
MethodsThe study included 143 consecutive patients referred for gated SPECT MPI with LVEF ≤50%. Gated SPECT MPI was performed according to a stress/rest protocol acquiring images with Tc 99m-tetrofosmin. Emory Cardiac Toolbox software was used for phase analysis and a standard deviation (SD) ≥43° was considered to indicate mechanical dyssynchrony.
ResultsMechanical dyssynchrony was present in 53.1% of the patients. Its predictors were diabetes (OR 2.0, p≤0.05), summed stress score (OR 1.1, p≤0.0005), summed rest score (OR 1.1, p≤0.0001), end-diastolic volume (OR 1.0, p≤0.0001), LVEF (OR 0.9, p≤0.0001), LVEF ≤35% (OR 3.1, p≤0.005) and LVEF ≤35% and QRS ≥120 ms (OR 3.5, p≤0.05). In this study QRS width and QRS ≥120 ms were not predictors of mechanical dyssynchrony.
ConclusionsMyocardial perfusion imaging can be used to assess mechanical dyssynchrony. In patients with impaired ventricular function mechanical dyssynchrony was highly prevalent and was related to parameters of left ventricular function and perfusion.
A avaliação de dessincronia ventricular mecânica através da análise do histograma de fase na cintigrafia de perfusão miocárdica tem sido descrita na literatura. Segundo a evidência atual, a presença de dessincronia mecânica encontra-se relacionada com a resposta à terapêutica de ressincronização.
ObjectivosAvaliar a presença e os preditores de dessincronia ventricular mecânica em pacientes com fração de ejeção ventricular esquerda (FEVE) ≤ 50%.
MétodosForam incluídos pacientes que realizaram cintigrafia de perfusão miocárdica e cuja FEVE foi inferior ou igual a 50%. A amostra estudada incluiu 143 pacientes. As imagens de perfusão foram adquiridas de acordo com o protocolo esforço seguido de repouso com Tc-99m Tetrafosmina. A avaliação de dessincronia baseou-se na determinação do desvio padrão obtido através da análise do histograma de fase (Emory Toolbox Software). A presença de dessincronia foi considerada quando o desvio-padrão no histograma de fase foi superior ou igual a 43°.
ResultadosA presença de dessincronia foi observada 53,1% dos doentes. Foram preditores de dessincronia, a diabetes (OR 2,0, p ≤ 0,05), o score de esforço (OR 1,1, p ≤ 0,0005), o score de repouso (OR 1,1, p ≤ 0,0001), o volume telediastólico (OR 1,0, p ≤ 0,0001), a FEVE (OR 0,9, p ≤ 0,0001), FEVE ≤ 35% (OR 3,1, p ≤ 0,005) e a presença em simultâneo de FEVE ≤ 35% e QRS ≥120 ms (OR 3,5, p ≤ 0,05). Nesta avaliação a duração do QRS ou a presença de QRS ≥ 120 ms não se relacionaram com a presença de dessincronia.
ConclusõesA cintigrafia de perfusão miocárdica pode ser usada na avaliação de dessincronia e esta tem uma elevada prevalência em pacientes com FEVE diminuída relacionando-se com parâmetros de função ventricular e perfusão miocárdica.
Heart failure (HF) is highly prevalent and represents a considerable social and economic burden.
Cardiac resynchronization therapy (CRT) shows benefits in patients with end-stage HF, leading to improvements in left ventricular (LV) end-diastolic diameter and LV ejection fraction (LVEF) after 6 months of follow-up in randomized trials. CRT also has a beneficial impact on mortality, functional class and quality of life in all HF patients.1–4
The current guidelines recommend CRT (class I, level of evidence A) in patients in NYHA class III or IV on optimal medical therapy, LVEF ≤35% and QRS duration ≥120 ms.5
In spite of the evidence in favor of CRT, 20–40% of HF patients do not respond to the procedure. It has been suggested that one reason could be the criterion used to define dyssynchrony, the QRS width, which is not necessarily related to mechanical dyssynchrony.6,7 Observational studies have shown that the presence of left ventricular mechanical dyssynchrony is associated with a favorable response to CRT with LV reverse remodeling, decreased mitral regurgitation and consequent improvement in clinical outcome. By contrast, the absence of significant mechanical dyssynchrony appears to be associated with a high rate of non-response to CRT.8–11
The use of phase analysis of gated single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) for the evaluation of mechanical dyssynchrony has been described in previous studies.12–15 Standard gated SPECT short-axis imaging enables detection of three-dimensional regional maximal counts for each time frame. The variation in regional maximal counts is proportional to regional wall thickening through the cardiac cycle. Information on mechanical contraction of all regions (phase distribution) of the LV is obtained and displayed as either a histogram or a polar map. In a normal LV, regional contraction starts almost at the same time, resulting in a uniform polar map and a narrow and peaked phase histogram. The standard deviation of the left ventricular phases (phase SD) is a quantitative parameter that describes the dispersion of LV regional contraction and is used to assess mechanical dyssynchrony. Significant dyssynchrony is defined, according to previous studies, as a phase SD ≥43°.12,14–16
The aim of this study was to assess the prevalence and predictors of mechanical dyssynchrony in patients with impaired ventricular function. As a secondary objective we set out to evaluate the predictors of mechanical dyssynchrony in patients with normal or mildly abnormal rest perfusion images.
MethodsStudy populationFrom February 1 to December 31 2011, consecutive patients referred for routine gated SPECT MPI in the Nuclear Medicine Department of Coimbra University Medical Center with estimated LVEF ≤50% were retrospectively included. Patients with atrial fibrillation or in pacemaker rhythm were excluded.
SPECT myocardial perfusion imagingGated SPECT MPI data were acquired according to a stress (exercise or pharmacological)/rest protocol using Tc-99m tetrofosmin and a dual-head (Ventri™) gamma camera. Perfusion defects were assessed visually and quantified using a 17-segment scoring system. The summed stress perfusion score (SSS), summed rest perfusion score (SRS) and summed difference score (SDS) were obtained. Patients were divided into two groups according to SRS and percentage of LV involvement: group 1 with <10% (normal or mildly abnormal images) and group 2 with ≥10% LV involvement (moderately to severely abnormal images). Gated SPECT MPI phase analysis was performed using the software in the Emory Cardiac Toolbox and an SD ≥43° was considered to indicate mechanical dyssynchrony.
Statistical analysisA descriptive analysis was performed considering:
- •
Clinical variables: age, gender, diabetes, hypertension, known CAD (previous myocardial infarction (MI) or percutaneous or surgical revascularization);
- •
ECG variables: QRS width, QRS ≥120 ms, bundle branch block pattern (BBB);
- •
SPECT MPI variables: exercise vs. pharmacological stress with adenosine, presence of perfusion abnormalities, SSS, SRS, SDS, LVEF, end-diastolic volume (EDV) and SD on phase analysis. Patients were divided into those with SRS <10% and those with SRS ≥10% of LV involvement.
Continuous variables were presented as mean ± standard deviation and categorical variables were presented as proportions. Continuous variables were compared using ANOVA or the nonparametric Mann–Whitney test when appropriate.
Fisher's exact test was used for categorical data. Considering SD ≥43° as the dependent variable, univariate logistic regression analysis was used to assess predictors of mechanical dyssynchrony. Significant predictors were then incorporated into multivariate models. To assess the influence of rest perfusion abnormalities on mechanical dyssynchrony, the previous analysis was performed in the patient group with normal or mildly abnormal rest perfusion images (SRS <10% of LV involvement). The level of statistical significance was taken to be 0.05. All statistical analyses were performed using StatView version 5.0.1 for Macintosh and Windows, SAS Institute.
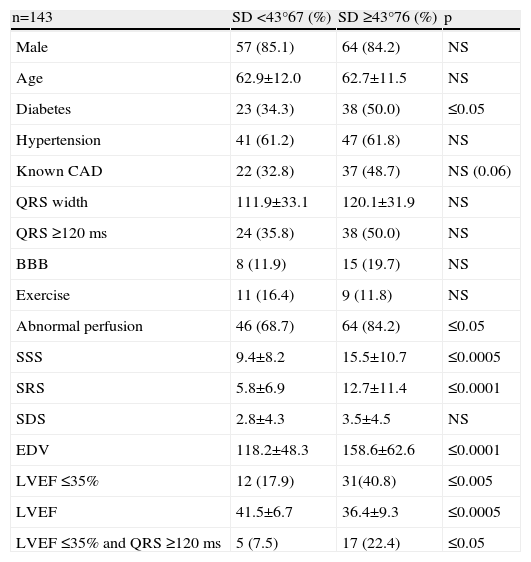
ResultsThe study included 143 patients, 22 women (15.4%) and 121 men (84.6%), with a mean age of 62.8±11.7 years. Clinical, electrocardiographic and SPECT MPI characteristics are shown in Table 1.
Clinical characteristics of the study population.
| Clinical variables | n=143 |
| Male | 84.6% |
| Age | 62.8±11.7 |
| Diabetes | 61 (42.7%) |
| Hypertension | 88 (61.5%) |
| Known CAD | 59 (41.3%) |
| ECG variables | |
| QRS width | 116.2±32.6 ms |
| QRS ≥120 ms | 62% (43.4) |
| BBB | 23 (16.1%) |
| Gated SPECT MPI variables | |
| Exercise | 20 (14.0%) |
| Abnormal perfusion | 110 (76.9) |
| SSS | 12.6±10.1 |
| SRS | 9.5±10.1 |
| SDS | 3.1±4.4 |
| EDV | 139.8±59.7 |
| LVEF | 38.8±8.7 |
| LVEF ≤35% | 43% (30.1) |
| SD | 46.1±20.3 |
| SD ≥43° | 76% (53.1) |
| SRS <10% LV | 71% (49.7) |
| QRS ≥120 ms and LVEF ≤35% | 22% (15.4) |
BBB: bundle branch block; CAD: coronary artery disease; EDV: end-diastolic volume; LVEF: left ventricular ejection fraction; SD: standard deviation; SDS: summed difference; SRS: summed rest score; SSS: summed stress score.
The prevalence of known CAD in this group was 41.3% and among these patients there was also a significant prevalence of hypertension and diabetes.
On baseline ECG a QRS width ≥120 ms was observed in 43.7% of the patients and a pattern of bundle branch block (BBB) in 16.1%.
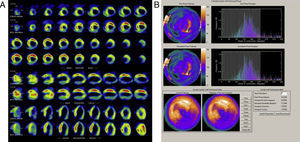
Almost all the exams were performed using pharmacological vasodilatation with adenosine (86%). A high number of abnormal perfusion images were seen (76.9%) and the prevalence of mechanical dyssynchrony was also high (53.1%). Figure 1 shows an example of a perfusion study with extensive defects and the related dyssynchrony analysis revealing mechanical dyssynchrony.
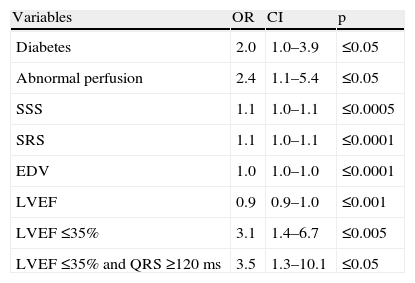
In Table 2 clinical, electrocardiographic and gated SPECT MPI characteristics are compared in patients with and without mechanical dyssynchrony according to SD.
Differences between patients with and without mechanical dyssynchrony.
| n=143 | SD <43°67 (%) | SD ≥43°76 (%) | p |
| Male | 57 (85.1) | 64 (84.2) | NS |
| Age | 62.9±12.0 | 62.7±11.5 | NS |
| Diabetes | 23 (34.3) | 38 (50.0) | ≤0.05 |
| Hypertension | 41 (61.2) | 47 (61.8) | NS |
| Known CAD | 22 (32.8) | 37 (48.7) | NS (0.06) |
| QRS width | 111.9±33.1 | 120.1±31.9 | NS |
| QRS ≥120 ms | 24 (35.8) | 38 (50.0) | NS |
| BBB | 8 (11.9) | 15 (19.7) | NS |
| Exercise | 11 (16.4) | 9 (11.8) | NS |
| Abnormal perfusion | 46 (68.7) | 64 (84.2) | ≤0.05 |
| SSS | 9.4±8.2 | 15.5±10.7 | ≤0.0005 |
| SRS | 5.8±6.9 | 12.7±11.4 | ≤0.0001 |
| SDS | 2.8±4.3 | 3.5±4.5 | NS |
| EDV | 118.2±48.3 | 158.6±62.6 | ≤0.0001 |
| LVEF ≤35% | 12 (17.9) | 31(40.8) | ≤0.005 |
| LVEF | 41.5±6.7 | 36.4±9.3 | ≤0.0005 |
| LVEF ≤35% and QRS ≥120 ms | 5 (7.5) | 17 (22.4) | ≤0.05 |
BBB: bundle branch block; CAD: coronary artery disease; EDV: end-diastolic volume; LVEF: left ventricular ejection fraction; SD: standard deviation; SDS: summed difference; SRS: summed rest score; SSS: summed stress score.
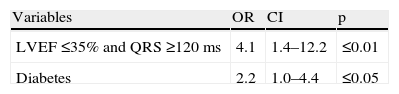
The main differences between patients with and without mechanical dyssynchrony were the presence of diabetes and gated SPECT MPI variables. Patients with mechanical dyssynchrony had a higher prevalence of abnormal perfusion exams, with larger fixed defects, higher EDV and worse LVEF. In spite of the differences observed in the prevalence of QRS ≥120 ms, this was not statistically significant. Twenty-two patients had LVEF ≤35% and QRS ≥120 ms, and 17 of these patients had mechanical dyssynchrony.
Using logistic regression analysis, the univariate predictors of mechanical dyssynchrony were diabetes, the presence of abnormal perfusion, the extent of stress/rest perfusion defects, EDV and LVEF dysfunction. The presence of LVEF ≤35% and QRS ≥120 ms was also a predictor of mechanical dyssynchrony (Table 3).
Univariate predictors of mechanical dyssynchrony.
| Variables | OR | CI | p |
| Diabetes | 2.0 | 1.0–3.9 | ≤0.05 |
| Abnormal perfusion | 2.4 | 1.1–5.4 | ≤0.05 |
| SSS | 1.1 | 1.0–1.1 | ≤0.0005 |
| SRS | 1.1 | 1.0–1.1 | ≤0.0001 |
| EDV | 1.0 | 1.0–1.0 | ≤0.0001 |
| LVEF | 0.9 | 0.9–1.0 | ≤0.001 |
| LVEF ≤35% | 3.1 | 1.4–6.7 | ≤0.005 |
| LVEF ≤35% and QRS ≥120 ms | 3.5 | 1.3–10.1 | ≤0.05 |
CI: confidence interval; EDV: end-diastolic volume; LVEF: left ventricular ejection fraction; OR: odds ratio; SRS: summed rest score; SSS: summed stress score.
Two multivariate models were built to identify variables that add incremental value over LVEF and QRS width, functioning as independent predictors of dyssynchrony.
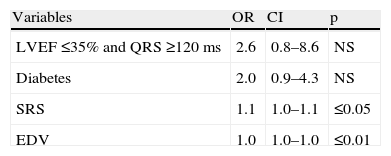
In the clinical model (Table 4), diabetes was an independent predictor of mechanical dyssynchrony. The model incorporating MPI variables (SRS and EDV) added predictive value to the clinical one and in this model SRS and EDV were independent predictors of dyssynchrony (Table 5).
Clinical and MPI model.
| Variables | OR | CI | p |
| LVEF ≤35% and QRS ≥120 ms | 2.6 | 0.8–8.6 | NS |
| Diabetes | 2.0 | 0.9–4.3 | NS |
| SRS | 1.1 | 1.0–1.1 | ≤0.05 |
| EDV | 1.0 | 1.0–1.0 | ≤0.01 |
Chi-square =28.5; p≤0.0001 (likelihood ratio). CI: confidence interval; EDV: end-diastolic volume; LVEF: left ventricular ejection fraction; OR: odds ratio; SRS: summed rest score.
According to these results, perfusion abnormalities, particularly those present at rest, were strong predictors of mechanical dyssynchrony. To assess how the extent of SRS affects mechanical dyssynchrony, the same analysis was performed in the patient group with normal or mildly abnormal rest images, excluding those with SRS corresponding to LV involvement ≥10%.
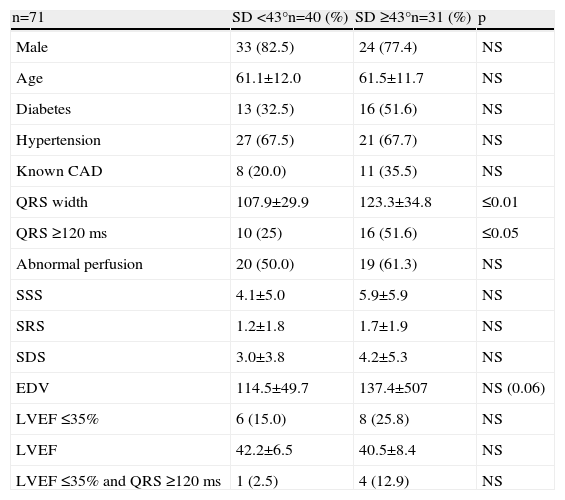
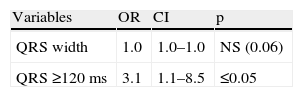
In these patients QRS width, and in particular QRS ≥120 ms, were significantly more prevalent in patients with SD ≥43°, and as expected QRS ≥120 ms was the only predictor of mechanical dyssynchrony in this group (Tables 6 and 7).
Differences between patients with and without mechanical dyssynchrony in patients with normal or mildly abnormal SRS.
| n=71 | SD <43°n=40 (%) | SD ≥43°n=31 (%) | p |
| Male | 33 (82.5) | 24 (77.4) | NS |
| Age | 61.1±12.0 | 61.5±11.7 | NS |
| Diabetes | 13 (32.5) | 16 (51.6) | NS |
| Hypertension | 27 (67.5) | 21 (67.7) | NS |
| Known CAD | 8 (20.0) | 11 (35.5) | NS |
| QRS width | 107.9±29.9 | 123.3±34.8 | ≤0.01 |
| QRS ≥120 ms | 10 (25) | 16 (51.6) | ≤0.05 |
| Abnormal perfusion | 20 (50.0) | 19 (61.3) | NS |
| SSS | 4.1±5.0 | 5.9±5.9 | NS |
| SRS | 1.2±1.8 | 1.7±1.9 | NS |
| SDS | 3.0±3.8 | 4.2±5.3 | NS |
| EDV | 114.5±49.7 | 137.4±507 | NS (0.06) |
| LVEF ≤35% | 6 (15.0) | 8 (25.8) | NS |
| LVEF | 42.2±6.5 | 40.5±8.4 | NS |
| LVEF ≤35% and QRS ≥120 ms | 1 (2.5) | 4 (12.9) | NS |
CAD: coronary artery disease; EDV: end-diastolic volume; LVEF: left ventricular ejection fraction; SD: standard deviation; SDS: summed difference; SRS: summed rest score; SSS: summed stress score.
This is a study of consecutive patients referred for gated SPECT MPI with impaired LVEF (≤50%). Our aim was to evaluate not only the prevalence of mechanical dyssynchrony in these patients but also its predictors among clinical, electrocardiographic and gated SPECT MPI variables. Mechanical dyssynchrony was defined, in accordance with previous studies, as a standard deviation, in phase analysis, of ≥43°.
The mean age of this group was 63 years, approximately 41% of the patients had a history of CAD (MI and/or myocardial revascularization) and there was also a significant prevalence of hypertension (62%) and diabetes (43%). Most of the patients thus had an intermediate to high risk of CAD.
In view of these findings, the high frequency of perfusion abnormalities found on gated SPECT MPI (77%) was not surprising. LVEF was ≤35% in 30.1% of the studied patients.
The precise prevalence of mechanical dyssynchrony in patients with impaired LVEF is still debated; although phase SD is a continuous variable it is used to dichotomize populations into normal vs. dyssynchronous using a cut-off value derived from data from a single center, with the inherent limitations. Even so, we used this value (43°) in our study, and a prevalence of 53% was found for mechanical dyssynchrony based on gated SPECT.17,18
The main differences between patients with and without mechanical dyssynchrony were the presence of diabetes and of an abnormal perfusion pattern (both higher in patients with mechanical dyssynchrony), LVEF (lower in patients with dyssynchrony), perfusion scores (with higher stress and rest scores and lower difference score in dyssynchrony patients) and EDV (higher in patients with mechanical dyssynchrony).
A wide QRS (≥120 ms) was more prevalent (although without statistical significance) in the dyssynchrony group (50%), but it should also be noted that among these patients, 50% had a narrow QRS. QRS duration was slightly greater in patients with dyssynchrony, but again without statistical significance. Several published studies have also found mechanical dyssynchrony in a considerable number of patients with a narrow QRS. The presence of ischemia and MI could be related to these findings.19 Whether these patients (with mechanical and without electrical dyssynchrony) benefit from CRT is still a matter of debate.20,21 Beshai et al. found no significant improvements with CRT in patients with narrow QRS.22,23
According to our results, there is a significant difference in LVEF (lower in patients with dyssynchrony) between the established groups. Dyssynchrony was identified in 72.1% of patients with LVEF ≤35% but also in 45.0% of those with LVEF between 35 and 50%. Similar findings have been described in previous studies.24 Atchey et al. found that 37% of patients with mild to moderate LVEF (35–50%) had significant degrees of mechanical dyssynchrony.25
The number of patients with LVEF and QRS criteria for CRT was also higher in the dyssynchrony group. Five of these patients (22.7%) did not demonstrate mechanical dyssynchrony. This result raises the issue of electrical without mechanical dyssynchrony; the existing evidence emphasizes the role of imaging techniques in the identification of the latter, and in the selection of patients who may benefit from CRT. It appears that CRT can correct mechanical dyssynchrony caused by electrical dyssynchrony.26–29
In univariate and multivariate logistic regression analysis, we found that in these patients the predictors of dyssynchrony were the presence of diabetes, the presence and extent of perfusion defects, especially rest score, EDV and LVEF. The simultaneous presence of wide QRS and low LVEF was also a predictor of mechanical dyssynchrony.
Diabetes is a powerful predictor of ischemic heart disease, so it was expected to find this relationship with mechanical dyssynchrony.30,31
Low LVEF and increased EDV are predictors of dyssynchrony. Low EF and an enlarged LV are accompanied by geometrical and morphological modifications and by conduction disturbances and changes in contraction patterns. These changes can potentially be reversed by CRT.32–34
We also found that the extent of perfusion defects, particularly of rest perfusion abnormalities, was also associated with increased dyssynchrony. This is not surprising considering that ischemic and infarcted segments exhibit significant degrees of motility changes (hypokinesia, akinesia or dyskinesia), resulting in abnormal contraction and dyssynchrony.35
Considering the presence of CRT criteria (QRS ≥120 ms and LVEF ≤35%), two models were built to identify independent predictors of mechanical dyssynchrony and the incremental values of adding these predictors to the conventional CRT criteria. In the model incorporating clinical and gated SPECT MPI variables, SRS and EDV were independent predictors of dyssynchrony and the incremental value of adding them to the CRT criteria was well demonstrated.
A study by Samad et al. had similar results but their study population had LVEF ≤35%. In these they found that QRS width was also an important predictor of mechanical dyssynchrony.36
In the study by Atchey et al. there was a weak correlation between QRS width and dyssynchrony in patients with LVEF between 35 and 50%.25 In our study, only 30.1% of patients had LVEF ≤35%, which could partly explain the lack of predictive value of QRS width in our population. It should also be borne in mind that these patients had a very high prevalence of perfusion abnormalities and known CAD, both of which lead to mechanical dyssynchrony. For this reason we also evaluated patients with a rest perfusion score under 10% of LV involvement. In these patients there was a clear relation between QRS width and the occurrence of mechanical dyssynchrony; in fact, QRS ≥120 ms was the only predictor of dyssynchrony. Rest perfusion abnormalities are associated with scar tissue, mechanical dyssynchrony and poor CRT response.37–40
Mechanical dyssynchrony has been evaluated by several methods in several contexts, and appears to be common not only in patients with impaired LVEF and wide QRS.18 According to the literature, mechanical dyssynchrony has additional value over QRS duration in predicting cardiac events in heart failure patients.41
LimitationsThis was an observational single-center cohort study with the limitations inherent to this kind of analysis. As symptoms of heart failure and functional class were not assessed, dyssynchrony could not be related to them, and this is an important constraint on further conclusions.
Conclusions- •
Far from questioning previous results and recommendations, this study corroborates the idea that mechanical dyssynchrony, according to established criteria, is highly prevalent in a population with impaired ventricular function and with a high prevalence of perfusion abnormalities.
- •
Mechanical dyssynchrony is very common in patients with normal QRS width and mildly abnormal LVEF.
- •
It was also found that clinical and MPI variables add value to the CRT criteria for predicting mechanical dyssynchrony.
- •
In patients with normal or mildly abnormal rest images, QRS width was the only predictor of mechanical dyssynchrony.
- •
In this context, gated SPECT myocardial perfusion imaging, which provides information on ischemia, fibrosis, ventricular function and dyssynchrony, is a valuable aid to therapeutic decision-making, including the use of cardiac resynchronization therapy.
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.