Hemophilia is the most common severe coagulation disorder (deficiency of factor VIII causing type A and deficiency of factor IX causing type B).1 Hemophilia-related changes in left ventricular (LV) function have been little examined. Three-dimensional (3D) speckle-tracking echocardiography (3DSTE) can provide detailed assessment of LV contractility represented by LV strains.2,3 The present study was designed to compare 3DSTE-derived LV strains between patients with hemophilia and matched controls.
The study analyzed 16 male patients with hemophilia, three of whom were excluded due to insufficient image quality. Eleven patients had hemophilia A, while two had hemophilia B. Mean age was 42.1±19.5 years. Hypertension, hypercholesterolemia, diabetes mellitus, HCV positivity and hemophilic arthropathy were present in five, three, two, nine and eight patients, respectively. All patients were treated with a 1000-6000 U/week dose of factors for each patient. Their results were compared to those of 15 healthy male controls with a mean age of 46.3±6.0 years.
Complete two-dimensional Doppler echocardiography (2DDE) and 3DSTE were performed in all hemophilia patients and controls. Results are from the MAGYAR-Path (Motion Analysis of the heart and Great vessels bY three-dimensionAl speckle-tRacking echocardiography in Pathological cases) Study.2 The institutional ethics committee at the University of Szeged approved the study, which complied with the 1975 Declaration of Helsinki (and updated versions) and all hemophilia patients and controls gave informed consent.
2DDE and 3DSTE were performed on a Toshiba Artida cardiac ultrasound system using a broadband 1-5 MHz PST-30SBP phased array transducer and a PST-25SX matrix array transducer (Toshiba Medical Systems, Tokyo, Japan), respectively. 2DDE-derived chamber quantifications and Doppler assessments were performed according to the guidelines.2,3 3DSTE was performed according to recent practice with offline analysis by 3D Wall Motion Tracking software as detailed previously. Global, mean segmental and regional LV strains in longitudinal (LS), circumferential (CS) and radial (RS) directions were calculated. Combined strains (area strain, AS, and 3D strain, 3DS) were also measured (Figure 1).3,4
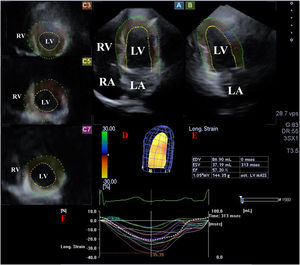
Images from three-dimensional (3D) speckle-tracking echocardiographic analysis in apical 4-chamber view (A), apical 2-chamber view (B) and apical (C3), mid-ventricular (C5) and basal (C7) left ventricular (LV) short-axis views together with a 3D virtual model of the LV (red D), LV volumetric data on the cardiac cycle (red E) and time – LV segmental and global strain curves (colored lines) with a time – LV volume changes curve (dashed line) during the cardiac cycle (red F). LA: left atrium; LV: left ventricle; RA: right atrium; RV: right ventricle.
2DDE data did not differ between hemophilia patients and controls (Table 1). Doppler echocardiography did not identify grade ≥1 valvular regurgitation or valvular stenosis in any patients or controls. 3DSTE-derived global and mean segmental LV strains did not show differences between groups, while only regional basal and midventricular LV circumferential strains (CSs) were significantly reduced in patients with hemophilia compared to healthy controls (Table 2).
Two-dimensional echocardiographic data of hemophilia patients and controls.
| Controls (n=15) | Hemophilia patients (n=13) | p | |
|---|---|---|---|
| LA diameter, mm | 40.0±4.1 | 38.6±3.5 | 0.3 |
| LV end-diastolic diameter, mm | 47.9±3.4 | 50.4±3.3 | 0.04 |
| LV end-diastolic volume, ml | 108.0±20.2 | 122.3±18.9 | 0.06 |
| LV end-systolic diameter, mm | 32.2±2.6 | 31.8±3.1 | 0.6 |
| LV end-systolic volume, ml | 38.4±7.2 | 40.8±9.8 | 0.4 |
| Interventricular septum, mm | 9.6±1.2 | 9.9±1.1 | 0.3 |
| LV posterior wall, mm | 9.4±1.1 | 9.8±1.1 | 0.4 |
| E, cm/s | 71.8±19.2 | 74.1±13.6 | 0.7 |
| A, cm/s | 62.1±15.5 | 65.6±13.4 | 0.5 |
| E/A | 1.20±0.35 | 1.08±0.28 | 0.3 |
| LV ejection fraction, % | 64.5±3.2 | 66.9±4.0 | 0.08 |
A: transmitral late diastolic inflow velocity; E: transmitral early diastolic inflow velocity; LA: left atrial, LV: left ventricular.
Comparison of three-dimensional speckle-tracking echocardiography-derived global and mean segmental left ventricular peak strains between hemophilia patients and controls.
| Controls (n=15) | Hemophilia patients (n=13) | |
|---|---|---|
| Global strains | ||
| RS, % | 25.5±10.4 | 21.2±9.9 |
| CS, % | -25.8±3.3 | -23.8±4.1 |
| LS, % | -15.6±2.3 | -15.8±3.4 |
| 3DS, % | 28.1±10.0 | 24.6±9.0 |
| AS, % | -38.3±3.3 | -36.9±4.9 |
| Mean segmental strains | ||
| RS, % | 28.0±10.0 | 23.9±10.3 |
| CS, % | -27.0±3.4 | -25.6±3.9 |
| LS, % | -16.5±2.1 | -14.6±4.9 |
| 3DS, % | 30.3±9.4 | 25.5±10.6 |
| AS, % | -39.4±3.1 | -38.2±5.0 |
| Regional strains | ||
| RS basal, % | 33.8±14.0 | 30.5±13.1 |
| RS mid, % | 31.0±12.8 | 26.6±11.3 |
| RS apex, % | 14.8±5.7 | 16.5±12.1 |
| CS basal, % | -26.2±5.8 | -22.4±2.8* |
| CS mid, % | -28.2±5.4 | -24.5±4.6* |
| CS apex, % | -26.2±8.9 | -30.0±10.2 |
| LS basal, % | -20.8±4.4 | -19.1±4.3 |
| LS mid, % | -13.1±4.6 | -12.6±4.8 |
| LS apex, % | -15.0±4.0 | -19.6±7.1 |
| 3DS basal, % | 36.9±13.5 | 34.7±13.9 |
| 3DS mid, % | 32.3±11.6 | 27.8±10.5 |
| 3DS apex, % | 17.1±6.5 | 17.7±12.2 |
| AS basal, % | -41.0±6.5 | -37.0±5.3 |
| AS mid, % | -38.4±6.0 | -34.7±6.2 |
| AS apex, % | -38.5±10.0 | -44.4±13.0 |
In a recent study, higher probability of cardiac disease, LV systolic dysfunction, coronary artery disease/microvascular disease, and LV electrical remodeling were detected in patients with hemophilia A compared to matched controls.5 Moreover, impaired myocardial LV systolic function represented by increased myocardial performance index has also been demonstrated, related to arterial stiffness, in these patients.6 Although the number of hemophilia patients examined in the present study was small, significant reductions in 3DSTE-derived regional LV CSs were observed. No global, mean segmental or other regional LV strains differed between the groups examined. This finding appears to be important in the context of previous results including reduced apical LV rotation and twist in hemophilia.7 Regional LV deformation abnormalities in hemophilia could be explained by various factors including hemophilia-related increased aortic stiffness, altered blood quality, accompanying risk factors, and others.6 However, further studies are warranted to confirm these findings.
Conflicts of interestThe authors have no conflicts of interest to declare.