Lipopolysaccharide (LPS) has been associated with myocardial inflammation, oxidative stress, apoptosis, and cardiac dysfunction, as well as death by causing sepsis. In this study, we investigated the effect of irbesartan (IRB), an angiotensin receptor antagonist, on cardiotoxicity caused by LPS.
MethodsThe experiment involved 24 Wistar albino rats divided into three groups of eight: control, LPS (5 mg/kg), and LPS (5 mg/kg)+IRB (3 mg/kg). Parameters including total oxidative status, total antioxidant status, oxidative stress index, and ischemia-modified albumin were measured to assess oxidative stress in heart tissues and serum. Serum CK, CK-MB, and LDH levels were measured spectrophotometrically. RT-qPCR was used to detect the mRNA expression levels of Bcl-2, BAX, p53, caspase-3, and sirtuin 1. Tissues taken from the heart and aorta were examined by immunohistochemistry and histopathology.
ResultsWhile there was an increase in the parameters indicating heart damage, oxidative stress, and apoptosis in the group given LPS, there was an improvement in all parameters and heart damage in the group treated with IRB.
ConclusionAs a result of our study, we determined that IRB has an ameliorating effect on myocardial damage caused by oxidative stress and apoptosis developed by the LPS-induced sepsis model.
O lipopolissacarídeo (LPS) tem sido associado à inflamação miocárdica, stress oxidativo, apoptose e disfunção cardíaca, bem como à morte por causar sépsis. Neste estudo, investigamos o efeito do irbesartan (IRB), um antagonista do recetor de angiotensina, contra a cardiotoxicidade causada pelo LPS.
MétodosA experiência envolveu 24 ratos albinos Wistar divididos em três grupos de oito: controlo, LPS (5 mg/kg) e LPS (5 mg/kg)+IRB (3 mg/kg). Parâmetros como estado oxidativo total (TOS), estado antioxidante total (TAS), índice de stress oxidativo (OSI) e albumina modificada por isquemia (IMA) foram medidos para avaliar o stress oxidativo nos tecidos cardíacos e no soro. Os níveis séricos de CK, CKMB e LDH foram medidos espectrofotometricamente. O RT-qPCR foi usado para detetar os níveis de expressão de mRNA de Bcl-2, Bax, p53, caspase-3 e Sirtuin 1. Os tecidos retirados do coração e da aorta foram examinados por imuno-histoquímica e histopatologia.
ResultadosEmbora tenha havido um aumento nos parâmetros que indicam lesão cardíaca, stress oxidativo e apoptose no grupo que recebeu LPS, foi determinado que houve uma melhoria em todos os parâmetros e no dano cardíaco no grupo tratado com IRB.
ConclusõesComo resultado do nosso estudo, determinamos que o IRB tem um efeito de melhoria na lesão miocárdica causada pelo stress oxidativo e apoptose desenvolvidos pelo modelo de sépsis induzida por LPS.
Inflammatory response syndrome, better known as sepsis, can result in death in intensive care units all over the world due to infection, immune system over-reaction, and multiple organ failure.1,2 Despite advances in health services, antibiotic therapy, and intensive care, mortality from sepsis remains high, and one of the major reasons for this is antibiotic resistance.3 The most frequent and serious complication of sepsis is cardiac dysfunction. Reactive oxygen species (ROS) are produced as a result of the activation of inflammatory pathways, which also causes oxidative and apoptotic damage to the myocardium and may result in myocardial dysfunction.4 Currently, specific biomarkers are used to accurately identify cardiac damage, but research into remediation strategies for sepsis-induced myocardial damage is still ongoing.
Lipopolysaccharide (LPS), a bacterial endotoxin, is a component of the cell wall of Gram-negative bacteria and can be used to induce sepsis in animals as well as to activate the septic response.5 It has been determined that the causes of myocardial damage after LPS administration are inflammation and an increase in cytotoxic free radicals, as well as activation of apoptosis mechanisms in cardiac cells.6,7
ROS and antioxidant enzyme systems are typically in balance in cells to prevent oxidative stress. Both exogenous and endogenous factors can produce ROS. Increased oxidative stress is caused by increased ROS in the environment and decreased antioxidant enzymes. Indicators for determining oxidative balance include total antioxidant status (TAS), total oxidant status (TOS), and oxidative stress index (OSI).8 The albumin cobalt binding test is used to measure ischemia-modified albumin (IMA), which is produced as a result of the modification of albumin by reactive oxygen derivatives created after ischemia and is one of the key indicators of oxidative stress. IMA was initially seen as a marker for myocardial ischemia, but it was later shown to rise with oxidative stress and other ischemic conditions.9 The increase in oxidative stress caused by inflammation and subsequent ROS production induces apoptosis, resulting in cell death.10
Increased caspase-3 (cas-3), Bcl-2-associated X protein (BAX) and p53, and decreased B-cell lymphoma 2 (Bcl-2) activity are all indicators of vascular apoptosis, which causes endothelial cell death.11,12 Sirtuin 1 (SIRT1) has been shown to protect against LPS-induced inflammation by down-regulating the p53-related apoptotic signaling pathway.13 Vascular endothelial growth factor (VEGF) has been found to be highly expressed in the myocardium as a result of various stimuli, such as inflammatory mediators, hypoxia, and ischemia. In rat ventricular monocytes, LPS has been shown to increase VEGF.14
Irbesartan (IRB), an angiotensin 1 receptor blocker, differs from other angiotensin receptor blockers, with a longer half-life, higher bioavailability, and lower plasma protein binding than losartan and valsartan.15 Furthermore, studies have confirmed that IRB has cardioprotective and renoprotective properties.16–18 IRB has been shown to protect against myocardial damage, but the exact mechanisms by which it does so are still unknown.19
ObjectivesIn this study we aimed to investigate the potential cardioprotective effects of IRB in the context of LPS-induced cardiotoxicity.
MethodsExperimental designTwenty-four adult female Wistar albino rats weighing 250–350 g were housed at 21–22°C and 60% humidity with a 12-hour light–12-hour dark cycle, and were given free access to standard commercial feed and water. Three groups of eight rats each (control, LPS, and LPS+IRB) were created at random.
In group 1 (control, n=8) 0.5 ml of saline was administered intraperitoneally (ip) from the right inguinal region. One hour after this application, 0.5 ml of saline was administered ip from the right inguinal region.
In group 2 (LPS, n=8), LPS (048K4126; Sigma-Aldrich, Stockholm, Sweden) was administered ip at a dose of 5 mg/kg and in a volume of 0.5 ml from the right inguinal region.20 Solid LPS was dissolved in 0.9% NaCl. One hour after LPS administration, 0.5 ml of saline was administered ip from the right inguinal region.
In group 3 (LPS+IRB, n=8) LPS at a dose of 5 mg/kg was administered ip in a 0.5 ml volume from the right inguinal region. One hour after LPS administration, 3 mg/kg IRB (Karvea, Sanofi-Aventis) was administered ip in a volume of 0.5 ml from the right inguinal region.21
Rats were sacrificed under anesthesia six hours after LPS administration using ketamine and xylazine. For biochemical analysis, blood samples were taken from the inferior vena cava and placed into biochemistry tubes containing gel. Before biochemical and genetic investigation, half of the left ventricles were kept at −80°C. The aorta and the remaining portion of the left ventricles were preserved in 10% buffered formalin for histological and pathological studies.
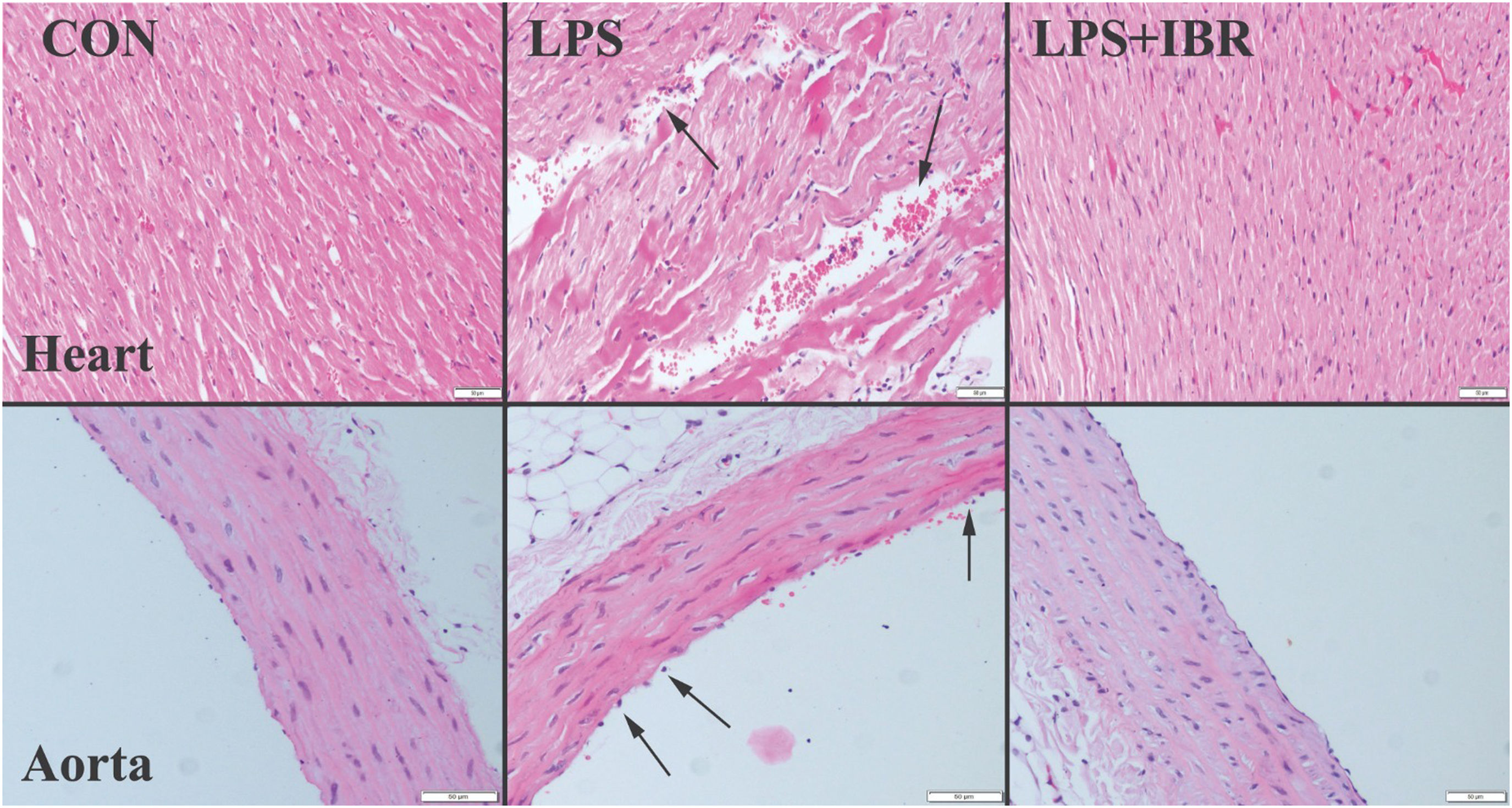
Histological analysisAfter being left at room temperature overnight, sections of 5 μm thickness were cut from paraffin blocks, stained with hematoxylin-eosin and examined under a microscope. Microscopic alterations of myocardial tissue were assessed based on the degree of hyperemia, hemorrhage, cloudy degeneration, muscle striation and necrosis. The severity of all histopathological findings was graded semiquantitatively depending on the degree and extent of the alteration, as follows: 0=normal, 1=mild, 2=moderate, and 3=severe. Ten areas from each animal were assessed under a 40× objective.
Immunohistochemical analysisSubsequently, slices from paraffin blocks were immunohistochemically stained for VEGF (JH121: sc-57496, 1/100 dilution) and cas-3 (anti-caspase-3 antibody [E-8]: sc-7272, 1/100 dilution) (Santa Cruz, Texas, USA) in accordance with the manufacturer's instructions. Following a 60-min incubation period with primary antibodies, the sections underwent immunohistochemistry analysis using the Mouse and Rabbit Specific HRP/DAB Detection IHC Kit (ab80436) (Abcam, Cambridge, UK). Negative controls used antigen dilution solutions instead of primary antibodies. Another pathologist also performed a blind review of the analyses.
For immunohistochemical analysis, sections were separately investigated for each antibody. To classify the severity of the immunohistochemical reaction of cells with markers, semiquantitative analysis was performed using a grading score ranging from 0 to 3 as follows: 0=negative, 1=focal weak staining, 2=diffuse weak staining, and 3=diffuse strong staining. For the assessment, 10 different areas in each section were examined under 40× objective magnification. Morphometric analyses and microphotography were performed using the cellSens Life Science Imaging software system (Olympus Co., Tokyo, Japan). The results were saved and statistically analyzed.
Biochemical analysisAfter the collected rat blood was centrifuged, serum was collected and kept at −80°C for later analysis. Serum creatine kinase (CK), creatine kinase-myocardial band (CK-MB), and lactate dehydrogenase (LDH) levels were measured spectrophotometrically (Beckman Coulter, USA). Bar-Or et al.’s method was used to calculate IMA levels to assess serum oxidative stress. The serum samples were spectrophotometrically analyzed at a wavelength of 470 nm, and the outcomes were reported as absorbance units.
Rat heart tissues weighing about 200 mg each were homogenized using an ULTRA-TURRAX T-25 homogenizer (Janke & Kunkel, IKA® Werke, Germany) using phosphate-buffered saline at a ratio of 1:9 (10 mM Na2HPO4, 1.8 mM KH2PO4, 2.7 mM KCl, 137 mM NaCl, pH 7.4). Homogenized heart tissues were then centrifuged for 10 min at 10000rpm. Using Erel's colorimetric method, the supernatants obtained after homogenization with an automatic analyzer (Beckman Coulter, USA) were used to calculate TAS and TOS values. The OSI value was then calculated using the formula OSI=[(TOS, μmol H2O2 equiv./l)/(TAS, mmol Trolox equiv./l)×100].18
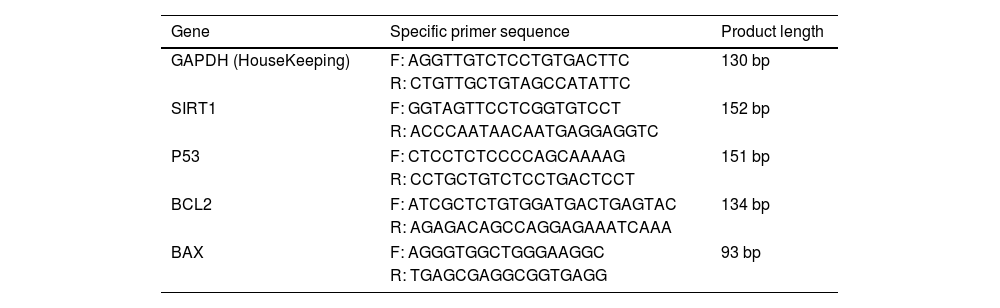
Real-time quantitative polymerase chain reaction analysisRNA was isolated from homogenized heart tissues with the GeneAll Ribospin RNA isolation kit (Thermo Fisher Scientific, USA) according to the manufacturer's protocol. The quantity and purity of RNA samples were determined using a NanoDrop device (Thermo Fisher Scientific, USA). The concentration of each isolated RNA sample was standardized at 500 ng/μl and they were stored at −80°C for use in the cDNA synthesis step. The A.B.T.™ cDNA Synthesis Kit (Atlas Biotechnology, Turkey) protocol was used for cDNA synthesis, which was carried out in a thermal cycler (Thermo Fisher Scientific, USA) with a total reaction volume of 20 μl for each sample. Specific mRNA sequences were found on the US National Center for Biotechnology Information website, and potential primer sequences were then tested (Table 1). The A.B.T.™ 2X real-time quantitative polymerase chain reaction (qPCR) SYBR-Green MasterMix (Atlas Biotechnology, Turkey) was used to assess expression levels using the CFX96 real-time qPCR instrument (Bio-Rad, CA, USA). Real-time qPCR conditions were established according to the manufacturer's instructions. Each sample was run in triplicate, and the GAPDH gene's expression was used to normalize the results.
Primers and product sizes for real-time qPCR assay.
| Gene | Specific primer sequence | Product length |
|---|---|---|
| GAPDH (HouseKeeping) | F: AGGTTGTCTCCTGTGACTTC | 130 bp |
| R: CTGTTGCTGTAGCCATATTC | ||
| SIRT1 | F: GGTAGTTCCTCGGTGTCCT | 152 bp |
| R: ACCCAATAACAATGAGGAGGTC | ||
| P53 | F: CTCCTCTCCCCAGCAAAAG | 151 bp |
| R: CCTGCTGTCTCCTGACTCCT | ||
| BCL2 | F: ATCGCTCTGTGGATGACTGAGTAC | 134 bp |
| R: AGAGACAGCCAGGAGAAATCAAA | ||
| BAX | F: AGGGTGGCTGGGAAGGC | 93 bp |
| R: TGAGCGAGGCGGTGAGG | ||
Bax: Bcl-2-associated X protein; BCL2: B-cell lymphoma 2; bp: base pairs; F: forward; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; R: reverse; SIRT1: sirtuin 1.
In the statistical analysis, the histological and immunohistochemical results, serum CK, CK-MB, LDH and IMA, tissue TAS, TOS, and OSI levels, as well as genetic parameters, were compared between the groups. The Shapiro-Wilk W test was used to determine normality of distributions. For the statistical analysis, one-way ANOVA with Bonferroni's test was used with GraphPad Prism v. 5 (GraphPad Software, Inc., La Jolla, CA, USA). The level of significance was set at p<0.05.
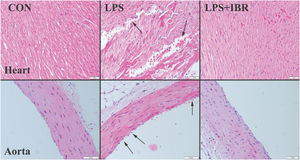
ResultsHistopathological findingsThe histopathological examination revealed normal heart architecture in the control group. The LPS group presented myocardial cell degeneration, minor hemorrhage, edema, and significant hyperemia, while the LPS+IRB group saw significant recovery after receiving IRB therapy.
Histological analysis of the aorta revealed normal tissue appearance, characterized by a uniformly stained and regularly arranged smooth endothelial layer, in the control group, as well as regular and wavy elastic fibers. The aortas of the LPS group showed loss of and injury to endothelial cells, as well as damage to elastic fibers. IRB markedly improved the histopathological findings in the aortas (Figure 1).
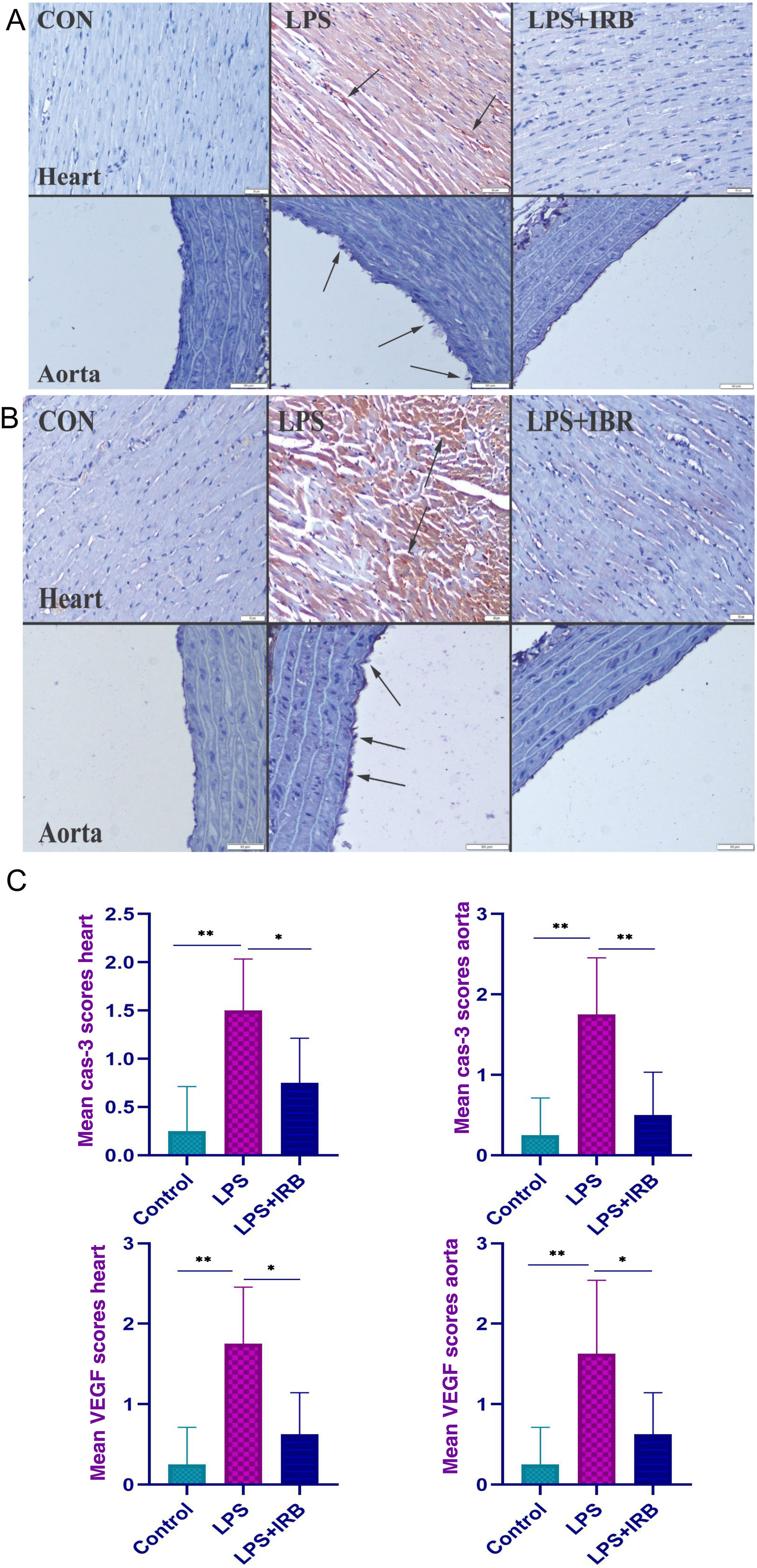
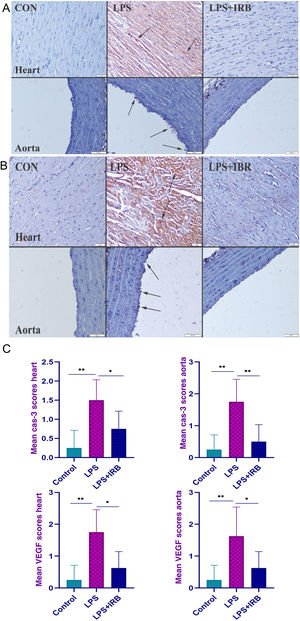
Immunohistochemical findingsImmunohistochemically, a marked increase in cas-3 and VEGF expression was observed in the LPS group, most highly expressed in endothelial and myocardial cells. IRB treatment caused marked amelioration in the LPS+IRB group (Figure 2A and B). The graph in Figure 2C depicts immunohistochemical scoring and statistical analysis of cas-3 and VEGF between groups.
Immunohistochemical findings for caspase-3 (A) and vascular endothelial growth factor (B) showing moderate myocardial hemorrhage (arrows) and endothelial cell loss (arrows) with elastic fiber damage in the aorta in the LPS group and scoring between groups (C). The differences between the means of the groups with different letter combinations were statistically significant (**p<0.001 and *p<0.05). Scale bars=50 μm; hematoxylin-eosin stain; streptavidin-biotin-peroxidase method. CON: control; LPS: lipopolysaccharide; LPS+IBR: lipopolysaccharide and irbesartan; VEGF: vascular endothelial growth factor.
Myocardial architecture was normal in the control group. In the LPS group, there was moderate myocardial hemorrhage and endothelial cell loss with elastic fiber damage in the aorta, while histopathological findings improved in the LPS-IRB group.
There was negative expression of VEGF in the control group but a marked increase in myocardial and endothelial cells in the LPS group, and decreased expression in the LPS+IRB group. The differences between the means of the groups with different letter combinations were statistically significant.
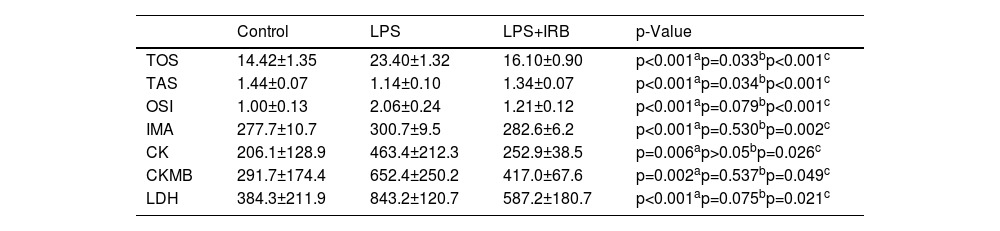
Biochemical findingsCK, CK-MB and LDH, serum levels of which rise as a result of cardiac injury, were significantly increased in the LPS group, while they decreased significantly in the IRB-treated group. TAS, TOS, and OSI levels were used to measure oxidative damage in tissues, with TAS levels being significantly lower in the LPS group compared to the control group, whereas TOS and OSI levels were significantly higher. TAS, TOS, and OSI levels in the LPS+IRB group were also close to those in the control group. In addition, we determined that while IMA, which is an indicator of serum oxidative stress, increased in the LPS group, it decreased significantly in the group treated with IRB (Table 2).
Biochemical parameters in heart tissue: mean values (± standard deviation) and statistical comparison between groups.
| Control | LPS | LPS+IRB | p-Value | |
|---|---|---|---|---|
| TOS | 14.42±1.35 | 23.40±1.32 | 16.10±0.90 | p<0.001ap=0.033bp<0.001c |
| TAS | 1.44±0.07 | 1.14±0.10 | 1.34±0.07 | p<0.001ap=0.034bp<0.001c |
| OSI | 1.00±0.13 | 2.06±0.24 | 1.21±0.12 | p<0.001ap=0.079bp<0.001c |
| IMA | 277.7±10.7 | 300.7±9.5 | 282.6±6.2 | p<0.001ap=0.530bp=0.002c |
| CK | 206.1±128.9 | 463.4±212.3 | 252.9±38.5 | p=0.006ap>0.05bp=0.026c |
| CKMB | 291.7±174.4 | 652.4±250.2 | 417.0±67.6 | p=0.002ap=0.537bp=0.049c |
| LDH | 384.3±211.9 | 843.2±120.7 | 587.2±180.7 | p<0.001ap=0.075bp=0.021c |
CK: creatine kinase; CKMB: creatine kinase MB; IMA: ischemia-modified albumin; IRB: irbesartan; LDH: lactate dehydrogenase; LPS: lipopolysaccharide; OSI: oxidative stress index; SD: standard deviation; TAS: total antioxidant status; TOS: total oxidant status.
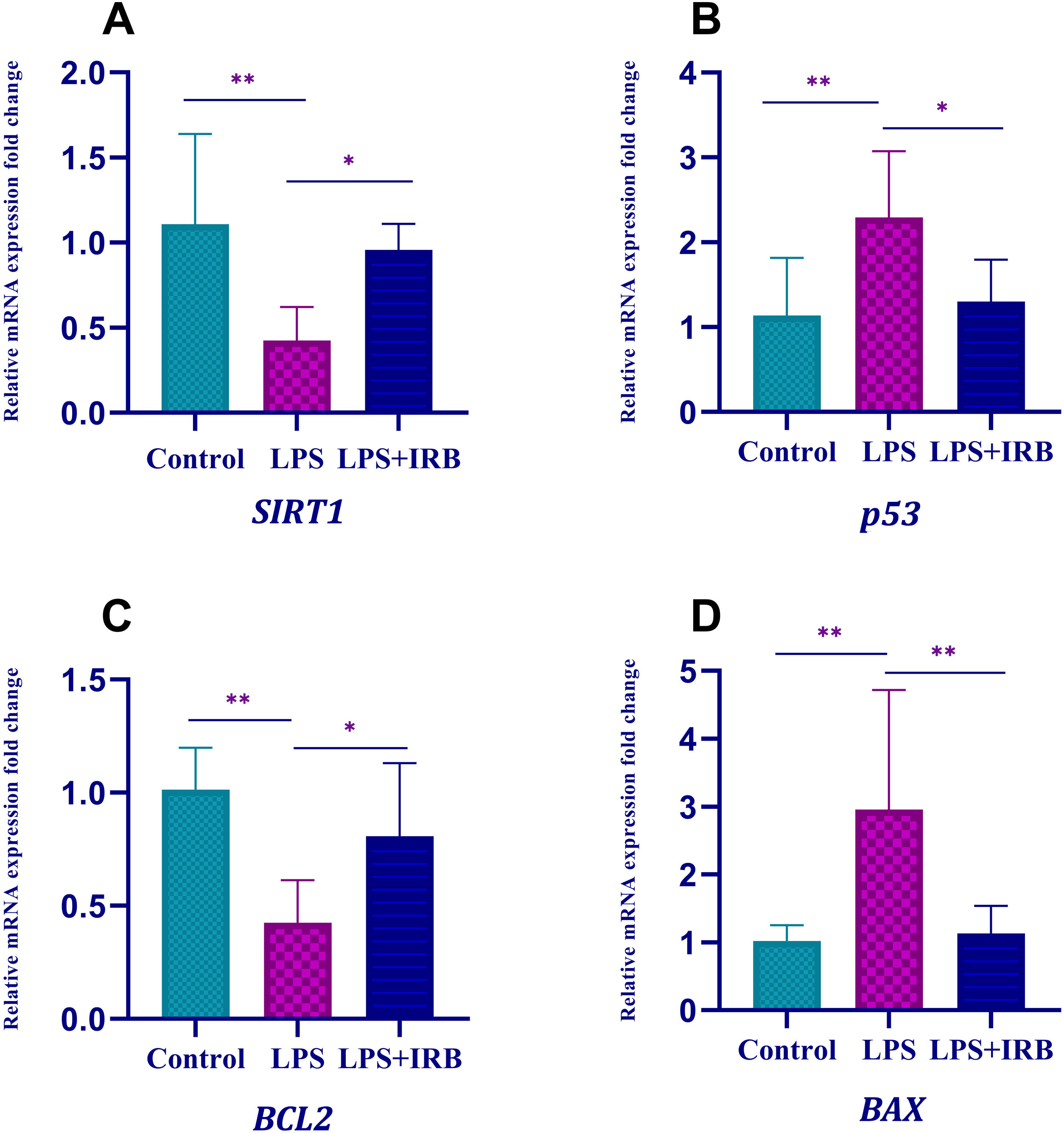
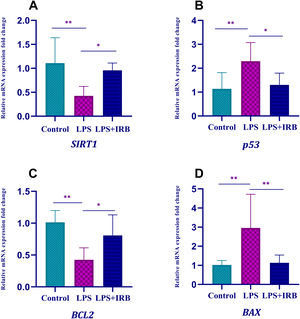
We found that expression of apoptotic genes (BAX and p53) increased and expression of antiapoptotic genes (SIRT1 and Bcl-2) decreased in the LPS group compared to the control group. After treatment with IRB, BAX and p53 expression decreased and SIRT1 and Bcl-2 expression increased significantly, to close to the control level (Figure 3).
The results of this study showed that LPS damages the heart and aorta, and IRB treatment ameliorates histopathological findings and also has a positive effect on heart damage by reducing oxidative stress and apoptotic effects.
DiscussionIn addition to causing systemic inflammation, LPS, a part of the bacterial cell wall, damages the brain, lungs, and heart through a variety of mechanisms,22 the three most significant of which are apoptosis, oxidative stress, and inflammation.23,24
Previous studies have revealed that inflammatory mediators and ROS production activated in LPS-induced sepsis cause depletion of antioxidants and an increase in oxidative stress, causing cardiotoxicity by damaging cellular lipids, proteins, and DNA, contributing to cardiac remodeling, apoptosis, and ultimately necrosis.25 In 2022, Skibska et al. showed that oxidative stress induced by LPS caused damage to rat ventricles and atria, resulting in a decrease in heart size and an increase in cardiac inflammatory and oxidative stress markers.26 A study by Khodir et al. demonstrated that the morphological and functional changes in the myocardium due to cardiotoxicity caused by LPS are clinically similar to myocardial infarction.27 In a 2012 study by Nako et al., it was shown that IRB ameliorates cardiac hypertrophy and fibrosis.28
In our study, the LPS group showed histopathological evidence of endothelial cell and elastic fiber loss, but there was a marked improvement in histopathological findings in the LPS+IRB group. Our histopathologic analysis showed that cardiotoxicity in the sepsis model we created was reduced as a result of IRB use, a finding that is compatible with previous studies.29–31 CK-MB, a specific biomarker of cardiac damage, was found to be significantly higher in the LPS group, which was consistent with the literature.32
Studies have shown that VEGF is released from ventricular myocytes in cases of LPS-induced sepsis and ischemia, increasing capillary permeability and playing an important role in the systemic inflammatory response.14,33 A 2020 study by Braile et al. concluded that VEGF increased LPS-induced inflammation.34 In our study, immunohistochemical analysis showed that VEGF was increased in both the heart and aorta in the LPS group, similar to previous studies, and decreased in both tissues after IRB treatment.
Studies have shown that oxidative stress parameters increase in LPS-induced inflammation and sepsis.3,28,35,36 According to a study by İlhan et al. published in 2022, IRB has a healing effect on oxidative stress parameters.18 Studies by Anjaneyulu et al. in 2004 and Al-Kuraishy et al. in 2019 revealed that IRB reduces oxidative stress.37,38 In our study, we determined that oxidative stress increased in heart tissue and blood in LPS-induced sepsis and decreased after treatment with IRB, which is consistent with the literature.
Inflammation and oxidative stress after sepsis induced by LPS eventually cause cell death. BAX, cas-3 and p53 are indicators of apoptosis, while Bcl-2 and SIRT1 are known to be antiapoptotic. Studies have shown that levels of BAX, cas-3, and p53 increase and Bcl-2 and SIRT1 decrease in LPS-induced sepsis and cardiomyocyte injury. It has also been suggested that SIRT1 can be used as a molecular biomarker for detecting sepsis.3,39 Studies on the ameliorative effect of IRB in the apoptosis process have shown that it decreases BAX and p53 levels and increases Bcl-2 levels.18,40 In addition, suppression of doxorubicin-induced cardiotoxicity by IRB was demonstrated in a study by El-Said et al.32 In our study, using immunohistochemistry, increases in BAX, p53 and cas-3 and decreases in SIRT1 and Bcl-2 were found in the LPS group. BAX, p53, and cas-3 levels decreased in the LPS+IRB group, while SIRT1 and Bcl-2 levels increased in a manner similar to the control group.41 The results of our study revealed that IRB has a healing effect by reducing apoptosis, which is consistent with previous studies. However, inflammatory pathways (such as nuclear factor kappa-B, interleukin 6, interleukin 1 beta and tumor necrosis factor alpha) were not investigated, which constitutes a limitation of our study. In addition, in the determination of gene expression, verification by western blot would have provided supporting evidence of the effect of IRB.
ConclusionIn our study, IRB showed a significant healing effect against cardiac damage in an LPS-induced sepsis model in rats. We found that parameters showing both oxidative damage (TAS, TOS, OSI, and IMA) and apoptotic damage (BAX, p53, Bcl-2, SIRT1, and cas-3) improved at levels close to the control group in rats given IRB after LPS-induced injury. We also found that VEGF, which increases inflammation, reached levels close to those in the control group after IRB treatment. These findings lead us to believe that IRB could be used to treat cardiac damage brought on by sepsis.
Authors’ contributionsLaboratory analyses: MYT, ÖÖ; experiment planning and implementation: HA, SC, MAS, NFK; histological imaging: ÖÖ; article writing and review: MYT, HA. The authors declare that all data were generated in-house and that no paper mill was used.
All of the authors contributed to the design of the study, collection of samples, analysis, and interpretation of data.
Ethical approvalThe animal experiments were approved by the local animal ethics committee of Suleyman Demirel University (Ethic No. 15.09.2022/06-79). The experiments were performed in accordance with the ARRIVE guidelines and EU Directive 2010/63/EU for animal experiments.
Conflicts of interestThe authors have no conflicts of interest to declare.