Vascular access site complications in transfemoral (TF) transcatheter aortic valve implantation (TAVI) are associated with increased morbidity and mortality; however, their incidence and predictors are conflicting between studies. This study sought to assess the incidence and predictors of vascular access site complications in patients undergoing TF TAVI.
MethodsA total of 140 patients undergoing TF TAVI were included in the study. Minimum iliofemoral diameter and iliofemoral calcium score (CS) were estimated from contrast-enhanced multidetector computed tomography imaging, using different thresholds according to aortic luminal attenuation. To assess the impact of the learning effect, the first 50% of TF TAVI procedures were compared to the remainder.
ResultsFifty-one patients presented access site complications (7.1% major, 29.3% minor), most of which were local bleeding or hematoma (11.4%), pseudoaneurysm (7.9%) or closure device failure (5.0%). In a multivariate logistic regression analysis that included sheath-to-iliofemoral artery ratio (SIFAR) (the ratio between the sheath outer diameter and minimum iliofemoral diameter), iliofemoral CS and center experience, SIFAR was the sole independent predictor of access site complications (hazard ratio 14.5, confidence interval [CI] 95% 1.75–120.12, p=0.013). The SIFAR threshold with the highest sum of sensitivity (71.4%) and specificity (53.4%) for access site complications was 0.92 (area under the curve 0.66, 95% CI 0.56–0.75, p=0.002).
ConclusionsVascular access site complications are frequent in patients undergoing TF TAVI. SIFAR was the only independent predictor of access site complications and therefore should be systematically assessed during pre-procedural imaging study.
As complicações do acesso vascular na implantação de válvula aórtica (TAVI) por via transfemoral (TF) foram associadas a um aumento da morbilidade e mortalidade; no entanto, a sua incidência e preditores não são consensuais. Este estudo procurou avaliar a incidência e os preditores de complicações do acesso vascular em doentes submetidos a TAVI por via TF.
MétodosForam incluídos 140 doentes. Foram determinados o diâmetro mínimo iliofemoral e o score de cálcio (ScCa) iliofemoral a partir de imagens de tomografia computorizada contrastadas, utilizando diferentes limiares de deteção de cálcio, de acordo com a atenuação luminal da aorta. Para avaliar o impacto da curva de aprendizagem, os resultados dos primeiros 70 procedimentos (50%) foram comparados com os restantes.
ResultadosUm total de 51 doentes apresentou complicações do acesso vascular (7,1% major, 29,3% minor), maioritariamente hemorragia/hematoma inguinal (11,4%), pseudoaneurisma (7,9%) e falência do dispositivo de encerramento (5,0%). Num modelo de regressão logística multivariado, que incluiu a razão diâmetro externo do introdutor/diâmetro iliofemoral mínimo (DEI/DIM), o ScCa iliofemoral e a experiência do centro, apenas a razão DEI/DIM foi preditor independente de complicações do acesso vascular (hazard ratio 14,5, IC 95% 1,75-120,12, p=0,013). O cut-off da razão DEI/DIM com a melhor combinação de sensibilidade (71,4%) e especificidade (53,4%) foi de 0,92 (AUC 0,66, IC 95% 0,56-0,75, p=0,002).
ConclusõesAs complicações do acesso vascular após TAVI por via TF são frequentes. A razão DEI/DIM foi o único preditor independente de complicações do acesso, devendo ser determinada sistematicamente durante a avaliação imagiológica pré-procedimento.
Transcatheter aortic valve implantation (TAVI) is nowadays a generally accepted treatment option for inoperable or high-risk patients with severe symptomatic aortic valve stenosis (AS).1–3 However, despite the notable developments in the technique, certain complications, such as those related to vascular access, are still common. The incidence of major vascular complications, according to the Valve Academic Research Consortium (VARC) definition, ranges between 5.0% and 23.3%.4 They are associated with increased procedural costs, prolonged hospitalization and increased mortality.5–7 Transfemoral (TF) access is the preferred access route for TAVI8 and most patients are eligible for this approach.9 Several variables have been identified as potential predictors of TF access complications, the most consistent being femoral artery diameter, arterial calcification and center experience.5,10–12 However, a standardized methodology to assess the vascular access site, in particular regarding calcification, is lacking.
This study sought to assess the incidence and predictors of vascular access site complications in patients undergoing TF TAVI.
MethodsStudy populationThis retrospective single-center study included all patients undergoing TF TAVI due to severe AS between August 2007 and November 2014. All patients were assessed by a multidisciplinary heart team consisting of clinical, imaging and interventional cardiologists, cardiac surgeons and anesthesiologists. Initial assessment included physical examination, baseline laboratory assays, transthoracic and transesophageal echocardiography, multidetector computed tomography (MDCT) of the aortic root and iliofemoral arteries, and invasive coronary angiography.
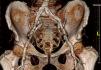
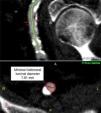
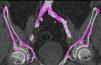
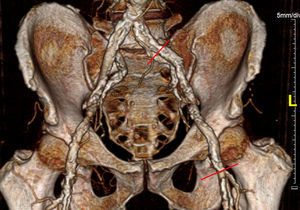
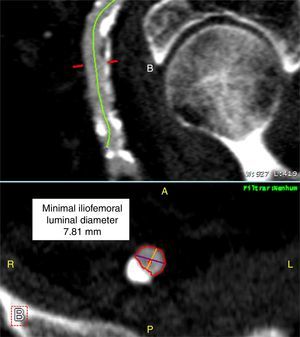
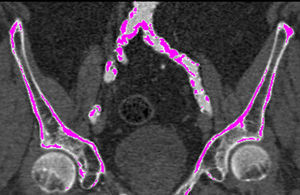
Iliofemoral vascular assessmentAll patients without severe renal dysfunction underwent contrast-enhanced MDCT (SOMATOM Sensation Cardiac 64, Siemens, Forchheim, Germany) for assessment of the access route. The scan parameters for this acquisition were tube voltage 100 kV, tube current modified according to patient size, rotation time 330 ms, slice collimation 0.6 mm and pitch 1.1. Images were reconstructed at a slice thickness of 0.75 mm. All patients received an intravenous injection of 80–100 ml of contrast agent (Ultravist 370, Bayer Schering Pharma, Berlin, Germany), followed by a 40 ml flush of normal saline solution, both at flow rates of 4.5 ml/s. Luminal attenuation (LA) was assessed by measuring the mean signal value (Hounsfield units [HU]) at the aortic root. Median LA was 446 HU (range 139–676 HU). Iliofemoral access was defined as the arterial segment composed of the external iliac artery and common femoral artery (Figure 1). The minimal luminal diameter (MLD) of the iliofemoral access was assessed using dedicated software (Aquarius iNtuition version 4.4.6, TeraRecon Inc., Foster City, CA) (Figure 2). The sheath-to-iliofemoral artery ratio (SIFAR) was defined as the ratio between the sheath outer diameter and iliofemoral MLD (in mm). The calcium score (CS) of the iliofemoral access was obtained from contrast-enhanced MDCT images using semi-automated software (syngo Calcium Scoring, Siemens Medical Solutions) and different calcium thresholds according to LA: 600 HU for LA <500 HU, 700 HU for LA 500–600 HU and 800 HU for LA >600 HU (Figure 3). These thresholds were empirically defined for this investigation. All MDCT scans and respective measurements were performed by experienced cardiac computed tomography readers.
ProcedureThe procedure was performed according to previously described techniques for TF TAVI with CoreValve (Medtronic Inc., Minneapolis, MN) and Edwards SAPIEN (Edwards Lifesciences, Irvine, CA) valves.13,14,1 The Edwards NovaFlex or Edwards eSheath introducer sheath (Edwards Lifesciences) was used for Edwards SAPIEN valve implantation. The sheath size was decided according to the manufacturer's recommendations for each valve size. An 18F Ultimum introducer (St. Jude Medical, Inc., St. Paul, MN) or an 18F Check-Flo adapter (Cook Medical, Inc., Bloomington, IN) was used for implantation of the third-generation CoreValve. Puncture of the common femoral artery was performed after iliofemoral angiography from the contralateral side. Since the beginning of our TAVI program a percutaneous closure technique has been used with one 10F Prostar XL or two Perclose ProGlide vascular closure systems (Abbott Vascular, Santa Clara, CA). The technical aspects of the vascular closure procedure with both systems have been previously described.15 All patients were pretreated with aspirin (100–150 mg/day) and clopidogrel (loading dose of 300 mg followed by 75 mg/day) and received unfractionated heparin during the procedure to achieve a target activated clotting time of 250–300 s.
Post-procedural careAll patients were admitted to the coronary care unit for at least the first 24 hours after the procedure, transitioning to an intermediate unit according to clinical course. Throughout hospitalization, the access site was frequently assessed and vascular Doppler echocardiography performed when there was any suspicion of complications.
Vascular access site complicationsVascular access site complications were classified according to the updated VARC criteria (VARC-2).16 They were generally managed by the interventional cardiologist when identified during the procedure or later during hospital stay by a vascular surgeon.
Learning effectTo assess the impact of the learning effect, the first 50% of TF TAVI procedures (early experience group) were compared to the remainder (late experience group).
Statistical analysisContinuous variables were described as means with standard deviations and categorical variables as frequencies and percentages. For normally distributed continuous variables an independent sampled t test was employed, whereas for non-normally distributed continuous variables a Mann-Whitney U-test was used. Chi-square analysis was used for comparisons of categorical variables. All variables with a p-value ≤0.1 in univariate analysis were included in a multivariate logistic regression analysis to identify the independent predictors of access site complications. Receiver operating characteristic (ROC) curve analysis was used to assess the SIFAR threshold with the best sum of sensitivity and specificity. A p-value <0.05 was considered statistically significant. All analyses were performed using SPSS statistics software (version 19.0, SPSS, Inc, Chicago, Illinois).
ResultsDuring the study period 170 patients underwent TAVI, of whom 140 were treated through the TF route. Baseline patient and procedural characteristics are summarized in Tables 1 and 2. Mean age was 78.3±9.1 years and 52.1% were female. In the early experience group, patients had a lower body mass index (26.5±5.0 vs. 28.3±5.1 kg/m2, p=0.041); other clinical characteristics were similar between the groups. A CoreValve was implanted in 67.1% of patients (third-generation CoreValve [n=91] and CoreValve Evolut R [n=3]) and an Edwards SAPIEN valve (Edwards SAPIEN XT [n=29] and Edwards SAPIEN 3 [n=17]) in the remainder. Almost all patients (98.6%) underwent an MDCT access route assessment. Right TF access was used in 80.0% of patients and an 18F sheath in 86.4%. Iliofemoral MLD was 7.28±1.39 mm and mean sheath outer diameter 6.87±0.43 mm, giving a SIFAR of 0.98±0.19. In 27 patients no calcium was identified in the iliofemoral access; mean iliofemoral CS was 256.6±582.9. Closure of the femoral artery access site was performed percutaneously using the Prostar XL device in 75.0% and Perclose ProGlide devices in 25.0%. In the late experience group, despite the trend to use smaller sheath sizes, there was a higher SIFAR (1.02±0.21 vs. 0.94±0.16, p=0.025). The use of Perclose ProGlide devices increased over time (45.7% vs. 4.3%, p<0.001).
Baseline patient characteristics.
| Total (n=140) | Early experience (n=70) | Late experience (n=70) | p | |
|---|---|---|---|---|
| Age (years) | 78.3±9.1 | 78.1±9.7 | 78.6±8.6 | 0.726 |
| Women | 73 (52.1%) | 35 (50.0%) | 38 (54.3%) | 0.612 |
| EuroSCORE II | 6.6±7.0 | 7.4±8.5 | 5.5±4.0 | 0.107 |
| NYHA III–IV | 104 (74.3%) | 47 (67.1%) | 57 (81.4%) | 0.068 |
| Body mass index (kg/m2) | 27.4±5.1 | 26.5±5.0 | 28.3±5.1 | 0.041 |
| Hypertension | 111 (79.3%) | 53 (75.7%) | 58 (82.9%) | 0.297 |
| Diabetes | 59 (42.1%) | 31 (44.3%) | 28 (40%) | 0.608 |
| Dyslipidemia | 95 (67.9%) | 45 (64.3%) | 50 (71.4%) | 0.366 |
| Coronary artery disease | 75 (53.6%) | 34 (48.6%) | 41 (58.6%) | 0.236 |
| Carotid artery disease | 18 (12.9%) | 12 (17.1%) | 7 (10.0%) | 0.217 |
| Peripheral artery disease | 16 (11.4%) | 10 (14.3%) | 6 (8.6%) | 0.288 |
| Anemia | 62 (44.3%) | 26 (37.1%) | 36 (51.4%) | 0.089 |
| GFR (ml/min/1.73 m2) | 58.1±24.9 | 57.6±26.7 | 58.7±22.5 | 0.822 |
| Aortic valve area (cm2) | 0.61±0.18 | 0.58±0.14 | 0.64±0.22 | 0.081 |
| Mean aortic valve gradient (mmHg) | 50.5±15.4 | 51.8±14.0 | 49.0±16.7 | 0.308 |
GFR: glomerular filtration rate; NYHA: New York Heart Association.
Procedural characteristics.
| Total (n=140) | Early experience (n=70) | Late experience (n=70) | p | |
|---|---|---|---|---|
| Transfemoral access | ||||
| Right side | 112 (80.0%) | 56 (80.0%) | 56 (80.0%) | 1.000 |
| 18F sheath | 121 (86.4%) | 70 (100%) | 51 (72.9%) | <0.001 |
| 14F sheath | 18 (12.9%) | 0 | 18 (25.7%) | <0.001 |
| Sheath outer diameter (mm) | 6.8±0.4 | 6.9±0.2 | 6.8±0.6 | 0.406 |
| SIFAR | 0.96±0.18 | 0.94±0.16 | 1.02±0.21 | 0.025 |
| CS | 256.6±582.9 | 195.1±292.5 | 311.7±752.3 | 0.258 |
| Percutaneous closure | ||||
| Prostar XL | 105 (75.0%) | 67 (95.7%) | 38 (54.3%) | <0.001 |
| Perclose ProGlide | 35 (25.0%) | 3 (4.3%) | 32 (45.7%) | <0.001 |
CS: calcium score; SIFAR: sheath-to-iliofemoral artery ratio.
According to the VARC-2 criteria, 51 patients (36.4%) presented vascular access site complications, which were major in 10 patients (7.1%) and minor in 41 (29.3%). The most common access site complications were local bleeding or hematoma (11.4%), pseudoaneurysm (7.9%) and closure device failure (5.0%) (Table 3). Almost half of patients who developed vascular access site complications were treated conservatively with external manual or mechanical compression, fluid therapy or blood transfusions. Most of the remaining cases were successfully treated with balloon or stent angioplasty. One patient underwent a retrograde recanalization technique to treat a left femoral artery occlusion and another underwent thromboembolectomy due to acute thromboembolic limb ischemia (Table 4). No patient required vascular surgery. The duration of hospital stay was 7.2±4.9 days and did not differ between patients who did and did not develop access site complications (p=0.574). The in-hospital mortality rate was 3.6%. Of these five deaths, one was directly related to vascular access site complication: hypovolemic shock resulting from retroperitoneal hemorrhage due to perforation of the iliac artery.
Vascular access site complications.
| Total | Major | Minor | |
|---|---|---|---|
| Bleeding/hematoma | 16 (11.4%) | 0 | 16 (11.4%) |
| Dissection | 2 (1.4%) | 0 | 2 (1.4%) |
| Rupture | 6 (4.3%) | 3 (2.1%) | 3 (2.1%) |
| Stenosis/occlusion | 6 (4.3%) | 0 | 6 (4.3%) |
| Pseudoaneurysm | 11 (7.9%) | 5 (3.6%) | 6 (4.3%) |
| Closure device failure | 7 (5.0%) | 0 | 7 (5.0%) |
| Distal embolization | 2 (1.4%) | 1 (0.7%) | 1 (0.7%) |
| Retroperitoneal hematoma | 1 (0.7%) | 1 (0.7%) | 0 |
Treatment of vascular access site complications.
| Total | Major | Minor | |
|---|---|---|---|
| Conservative | 25 (17.9%) | 3 (2.1%) | 22 (15.7%) |
| Balloon angioplasty | 9 (6.4%) | 1 (0.7%) | 8 (5.7%) |
| Stenting | 14 (10%) | 5 (3.6%) | 9 (6.4%) |
| Retrograde recanalization | 1 (0.7%) | 0 | 1 (0.7%) |
| Thrombin injection | 1 (0.7%) | 0 | 1 (0.7%) |
| Thromboembolectomy | 1 (0.7%) | 1 (0.7%) | 0 |
Univariate and multivariate logistic regression analysis for prediction of vascular access site complications are represented in Table 5. In a multivariate analysis that included factors with p<0.1 in univariate analysis (SIFAR, iliofemoral CS and center experience), SIFAR was the sole independent predictor of vascular access site complications (hazard ratio [HR] 14.5, 95% confidence interval [CI] 1.75–120.12, p=0.013). The SIFAR threshold with the highest sum of sensitivity (71.4%) and specificity (53.4%) for access site complications was 0.92 (area under the curve 0.66, 95% CI 0.56–0.75, p=0.002).
Univariate and multivariate logistic regression analysis for access site complications.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age | 1.00 (0.96–1.03) | 0.780 | ||
| Female | 1.19 (0.60–2.38) | 0.621 | ||
| EuroSCORE II | 1.00 (0.94–1.05) | 0.858 | ||
| Body mass index | 1.01 (0.95–1.08) | 0.692 | ||
| Diabetes | 0.94 (0.47–1.89) | 0.861 | ||
| Peripheral arterial disease | 1.88 (0.66–5.37) | 0.236 | ||
| GFR <60 ml/min/1.73 m2 | 1.00 (0.46–2.17) | 1.000 | ||
| SIFAR | 17.78 (2.41–130.88) | 0.005 | 14.50 (1.75–120.12) | 0.013 |
| Iliofemoral CS | 1.00 (1.00–1.00) | 0.097 | 1.00 (1.00–1.00) | 0.101 |
| Prostar XL | 0.83 (0.38–1.82) | 0.639 | ||
| Late experience group | 1.86 (0.93–3.78) | 0.079 | 1.37 (0.63–2.97) | 0.429 |
CI: confidence interval; CS: calcium score; GFR: glomerular filtration rate; HR: hazard ratio; SIFAR: sheath-to-iliofemoral artery ratio.
This study shows that vascular access site complications are still common among patients undergoing TF TAVI. We report an overall access site complication rate of 36.4%, higher than those described by other centers, which range between 5.0% and 23.3%.4 This is mainly due to a higher rate of minor complications (29.3%), in particular local bleeding or hematoma, which were treated conservatively in most cases. Previous investigations showed that minor vascular complications were not associated with increased hospital stay or with increased 30-day mortality.5
Several parameters have been indicated as potential predictors of access site complications. In our study, as well as clinical and procedural variables we assessed diameter and calcification of iliofemoral access by MDCT. SIFAR reflects both sheath size and iliofemoral MLD and was shown to be the only independent predictor of overall access site complications. We found that the best SIFAR threshold to predict access site complications was 0.92. This threshold is slightly more restrictive compared to other studies,5,17 probably because we considered prediction of both major and minor complications. In a prospective study of 130 TAVI patients, Hayashida et al. found that a SIFAR threshold of 1.05 (assessed by angiography or MDCT) predicted a higher rate of VARC major complications and 30-day mortality.5 MDCT is the preferred method to determine iliofemoral luminal diameter,18 however angiography has shown a good correlation with measurements obtained by MDCT.17 The predictive value of other variables, such as minimal luminal area or diameter derived from minimal luminal area, has never been assessed.
Some reports demonstrate that iliofemoral calcification is an important predictor of major vascular complications in patients undergoing TF TAVI.5,17,19 In these studies iliofemoral calcification was graded semi-quantitatively (from 0 – no calcification – to 3 – marked calcification) by MDCT or angiography. To our knowledge, this is the first study in which iliofemoral calcification was assessed quantitatively. We defined empirical thresholds to determine iliofemoral CS in contrast-enhanced MDCT images and found that the total amount of calcium in the iliofemoral segment was not a predictor of access site complications. Recently, Reinthaler et al. showed that iliofemoral calcification assessed semi-quantitatively by MDCT or angiography was not a predictor of vascular complication in a TF TAVI population, but the presence of circumferential calcification (>75%) assessed by MDCT was.17
In the late experience group there was an unexpected trend toward a higher access site complication rate compared to the early experience group. However, when adjusted for other variables in the multivariate analysis this trend was no longer present (p=0.429). This is mainly explained by the treatment of patients with progressively higher SIFAR by TF access (0.94±0.16 in early experience vs. 1.02±0.21 in late experience, p=0.025). In addition, during our TAVI program we gradually changed from Prostar to ProGlide closure devices, which could have nullified the benefits of the greater experience with the former. These differences between the first and second halves preclude an accurate assessment of the learning effect, however most other studies reported a significant reduction in the rate of vascular complications with increasing experience.5,11,12
Based on our findings, we expect a reduction in vascular access site complications with the introduction of progressively smaller delivery systems.
Study limitationsThis is a retrospective single-center study with a mixed cohort of recipients of self-expandable and balloon expandable valves. The small sample size does not allow assessment of independent predictors of major access site complications only and their impact on morbidity and mortality.
Other factors that could lead to access site complications, such as the degree of vessel tortuosity and of circumferential calcification, were not analyzed in this study.
ConclusionsVascular access site complications are frequent in patients undergoing TF TAVI. SIFAR was the only independent predictor of access site complications and therefore should be systematically assessed during pre-procedural imaging study.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.